Effects of biological soil crusts on soil enzyme activities of Artemisia ordosica community in the Mu Us Desert of northwestern China
-
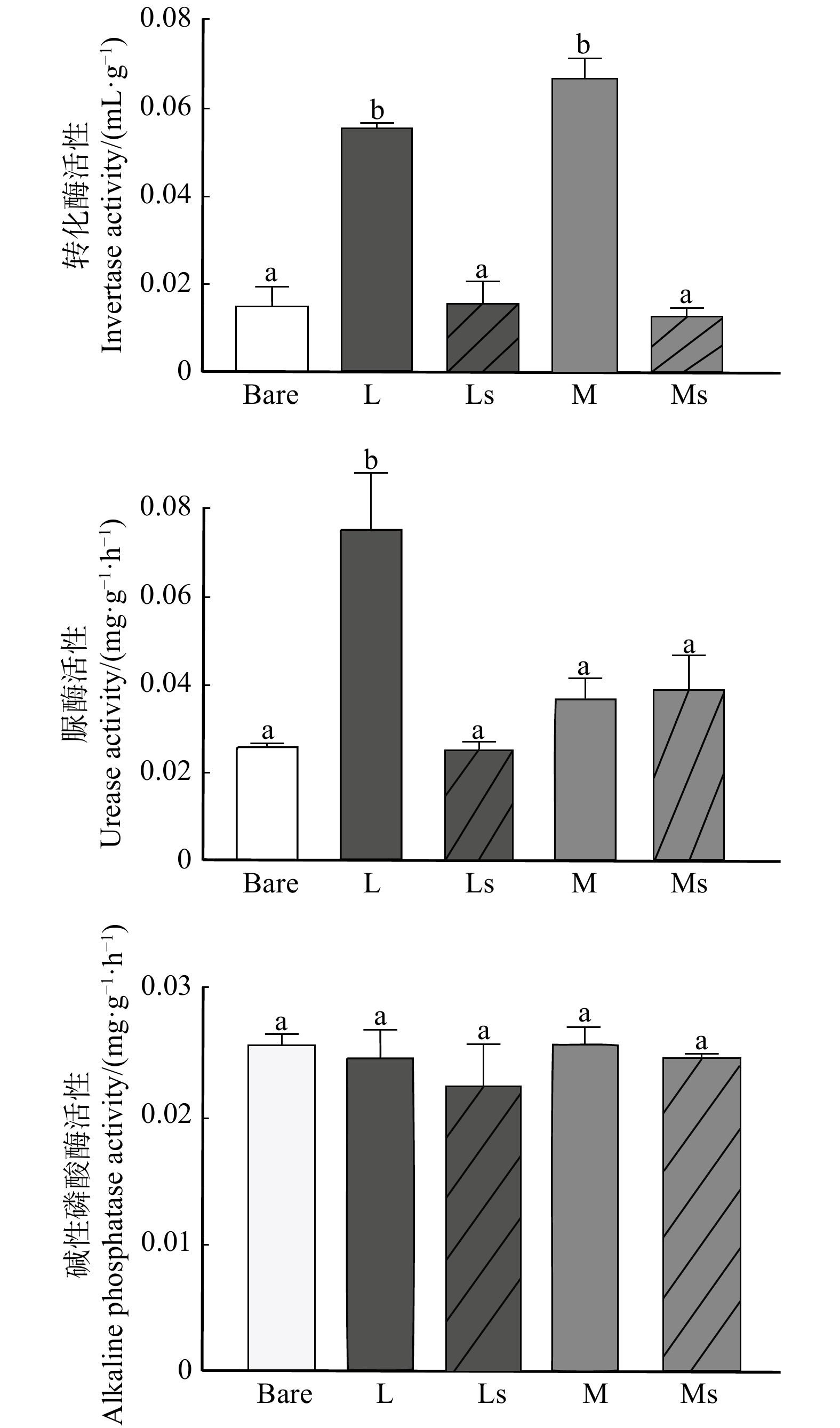
摘要:目的 通过研究生物土壤结皮对毛乌素沙地油蒿群落土壤酶活性的影响,探究半干旱区不同类型生物土壤结皮对土壤养分周转的作用,为认识生物土壤结皮对沙地植被恢复的影响提供理论参考。方法 以宁夏盐池毛乌素沙地油蒿灌丛间的裸地、地衣结皮和苔藓结皮为研究对象,分析油蒿灌丛间裸地土壤(0 ~ 5 cm)、地衣结皮层和苔藓结皮层(0 ~ 1 cm)及其下层土壤(1 ~ 5 cm)的土壤理化性质和土壤酶活性的变化特征。结果 (1)地衣结皮层和苔藓结皮层均显著改善油蒿灌木群落的土壤理化性质,且苔藓结皮层改善作用更为明显。地衣结皮层和苔藓结皮层相比油蒿灌丛间裸地,土壤有机碳含量(SOC)分别提高3.30倍和6.51倍,微生物量碳含量(MBC)分别显著提高2.79倍和6.58倍,微生物量氮含量(MBN)分别显著提高3.49倍和12.73倍,全氮含量(TN)分别提高2.67倍和4.46倍,全磷含量(TP)分别显著提高1.82倍和2.06倍。生物土壤结皮下层土壤与灌丛间裸地相比微生物量氮(MBN)、TN和TP之间无显著差异,MBC显著降低。(2)与油蒿灌丛间裸地相比,地衣结皮层和苔藓结皮层显著提高了土壤转化酶和脲酶活性,其中,地衣结皮层分别提高转化酶和脲酶活性3.58倍和2.80倍。苔藓结皮层显著提高转化酶活性4.23倍。碱性磷酸酶活性则无显著影响。另外,生物土壤结皮层下土壤3种酶活性均无显著提高。(3)土壤理化性质显著影响3种土壤酶活性。SOC、MBC、MBN、TN和TP与土壤转化酶活性呈显著正相关,pH与土壤转化酶活性显著负相关。SOC、MBN和TP与土壤脲酶活性显著正相关。pH与土壤碱性磷酸酶活性显著负相关。结论 地衣结皮和苔藓结皮均能加速油蒿灌丛土壤碳素周转;氮素周转则主要由地衣结皮调控。另外,仅结皮层能提高油蒿群落养分周转。研究结果表明,生物土壤结皮可以加速油蒿群落间土壤养分的周转和提高土壤质量,促进该区域植被和荒漠生态系统的恢复。Abstract:Objective We studied the effects of biological soil crusts (BSCs) on soil enzyme activities in Artemisia ordosica community of Mu Us Desert, northwestern China, aiming to explore the effects of different types of biological soil crusts on soil nutrient turnover in arid and semi-arid area, which could provide theoretical basis for understanding the effects of biological soil crusts on vegetation restoration.Method We took soil between Artemisia ordosica community (bare land), lichen crust layer, moss crust layer and its underlying soil as objectives, and analyzed their physical and chemical properties and soil enzyme activities.Result (1) Both the lichen crust layer and the moss crust layer significantly improved the soil physical and chemical properties, which were highest in moss crust layer compared with bare land and lichen crust layer. Compared with bare land, soil organic carbon content (SOC) in lichen crust layer and the moss crust layer increased by 3.30 times and 6.51 times, microbial biomass carbon content (MBC) increased by 2.79 times and 6.58 times, microbial biomass nitrogen content (MBN) increased by 3.49 times and 12.73 times, soil total nitrogen content (TN) increased by 2.67 times and 4.46 times, and total phosphorus content (TP) increased by 1.82 times and 2.06 times, respectively. There was no significant difference in microbial biomass nitrogen (MBN), TN and TP among the underlying soil in lichen crust layer, moss crust layer and bare land. The MBC content was significantly decreased in underlying soil of lichen crust layer and moss crust layer compered with bare land. (2) The invertase and urease activities in lichen crust layer and the moss crust layer were higher than bare land. Compared with bare land, the invertase and urease activities in lichen crust layer and moss crust layer were significantly increased by 3.58 times and 2.80 times, respectively, and the invertase activity was significantly increased by 4.23 times in moss crust layer. There were no significant differences among the underlying soil in lichen crust layer, moss crust layer and bare land on the three enzyme activities. There were no significant differences on alkaline phosphatase activity between the biological soil crusts and bare land. (3) Soil physicochemical properties significantly affected the three soil enzyme activities. SOC, MBC, MBN, TN and TP were significantly positively correlated with soil invertase activity, and pH was significantly negatively correlated with soil invertase activity. SOC, MBN and TP were significantly positively correlated with soil urease activity. pH was significantly negatively correlated with soil alkaline phosphatase activity.Conclusion Both lichen crust and moss crust can improve the carbon transformation in Artemisia ordosica community; however, the nitrogen transformation in Artemisia ordosica community was mainly regulated by lichen crust. In addition, only the biological soil crust layer could improve the nutrient turnover in Artemisia ordosica community. The results show that biological soil crust can accelerate the turnover of soil nutrients and improve soil quality, which are conducive to the restoration of vegetation and desert ecosystems.
-
Keywords:
- Mu Us Desert /
-
Artemisia ordosica community / - lichen crust /
- moss crust /
- soil enzyme activity
-
-
图 1 油蒿灌丛间裸地土壤、生物结皮层及层下土壤酶活性
数据为平均值 ± 标准误,不同字母表示样品间土壤酶活性之间存在显著差异(n = 3; P < 0.05)。Bare. 裸地;L. 地衣结皮层;Ls. 地衣结皮层下土壤;M. 苔藓结皮层;Ms. 苔藓结皮层下土壤。Data represent mean ± SE, different letters indicate significant differences in soil enzyme activities among samples (n = 3; P < 0.05). Bare, bare land; L, lichen crust layer; Ls, soil beneath lichen crust layer; M, moss crust layer; Ms, soil beneath moss crust layer.
Figure 1. Soil enzyme activities of soil among bare land, biological soil crusts and the underneath soil in A. ordosica shrub
表 1 样地基本概况
Table 1 Basic information of sample plots
样地
Sample plot坡度
Slope/(°)结皮特征
Caracteristics of biological soil crusts (BSCs)主要植物种
Main plant species植被盖度
Vegetation coverage/%样地1
Sample plot 13 生物结皮总盖度95%,地衣结皮相对盖度45%, 苔藓结皮相对盖度40%
Total coverage of BSCs is 95%, relative coverage of lichen crust is 45%, relative coverage of moss crust is 40%油蒿 Artemisia ordosica
细叶小苦荬 Ixeridium gracile
虫实 Corispermum mongolicum
沙鞭 Psammochloa villosa85 样地2
Sample plot 27 生物结皮总盖度85%,地衣结皮相对盖度50%,苔藓结皮相对盖度40%
Total coverage of BSCs is 85%, relative coverage of lichen crust is 50%, relative coverage of moss crust is 40%油蒿 Artemisia ordosica
细叶小苦荬 Ixeridium gracile
刺藜 Chenopodium aristatum75 样地3
Sample plot 35 生物结皮总盖度85%,地衣结皮相对盖度40%, 苔藓结皮相对盖度45%
Total coverage of BSCs is 85%, relative coverage of lichen crust is 40%, relative coverage of moss crust is 45%油蒿 Artemisia ordosica
细叶小苦荬 Ixeridium gracile
虫实 Corispermum mongolicum
沙生针茅 Stipa glareosa80 表 2 油蒿灌丛间裸地、生物结皮层及层下土壤理化性质
Table 2 Physicochemical properties of soil among bare land, biological soil crusts, and the underneath soil in Artemisia ordosica shrub
理化性质
Physicochemical property裸地
Bare land地衣结皮层
Lichen crust layer地衣结皮层下土壤
Soil underlying lichen crust layer苔藓结皮层
Moss crust layer苔藓结皮层下土壤
Soil underlying moss crust layerpH 8.24 ± 0.03a 8.17 ± 0.23a 8.79 ± 0.12b 7.66 ± 0.06c 8.33 ± 0.02a SWC/% 0.64 ± 0.21ab 0.25 ± 0.06a 1.00 ± 0.18ab 2.25 ± 0.57c 1.77 ± 0.17b SOC/(g·kg−1) 2.66 ± 0.66a 8.77 ± 1.61b 3.05 ± 0.96a 17.33 ± 1.09c 4.59 ± 0.37a MBC/(mg·kg−1) 206.47 ± 13.87ab 575.81 ± 152.50b 65.06 ± 43.60a 1 441.02 ± 180.14c 84.68 ± 18.84a MBN/(mg·kg−1) 17.83 ± 1.40a 62.27 ± 14.32b 17.22 ± 7.32a 226.99 ± 30.80c 21.70 ± 1.94ab TN/% 0.03 ± 0.00a 0.07 ± 0.01ab 0.02 ± 0.00a 0.11 ± 0.04b 0.03 ± 0.00a TP/(g·kg−1) 0.15 ± 0.00a 0.28 ± 0.04ab 0.23 ± 0.05a 0.32 ± 0.02b 0.24 ± 0.02a 注:数据表示为平均值 ± 标准误,不同小写字母表示样品间土壤理化性质差异性显著(n = 3,P < 0.05)。SWC. 土壤含水量;SOC. 土壤有机碳含量;TN. 土壤全氮含量;TP. 土壤全磷含量;MBC. 土壤微生物量碳含量;MBN. 土壤微生物量氮含量。下同。Notes: data represent the mean ± SE (n = 3). Varied lowercase letters indicate significant differences in soil physicochemical properties among samples (n = 3,P < 0.05). SWC, soil water content; SOC, soil organic carbon content; TN, total nitrogen content; TP, total phosphorus content; MBC, soil microbial biomass carbon content; MBN, soil microbial biomass nitrogen content. The same below. 表 3 土壤理化性质对土壤酶活性的相关性分析
Table 3 Correlation analysis between soil properties and soil enzyme activities
理化性质
Physicochemical property转化酶活性
Invertase activity脲酶活性
Urease activity碱性磷酸酶活性
Alkaline phosphatase activitypH −0.845 −0.059 −0.588 SWC 0.186 0.381 0.084 SOC 0.908 0.371 0.376 MBC 0.922 0.391 0.347 MBN 0.883 0.405 0.297 TN 0.868 0.412 0.285 TP 0.763 −0.652 0.294 注:黑体表示相关性显著(n = 3;P < 0.05)。Note: boldface indicates a significant correlation (n = 3; P < 0.05). -
[1] 黄建平, 季明霞, 刘玉芝, 等. 干旱半干旱区气候变化研究综述[J]. 气候变化研究进展, 2013, 9(1):9−14. Huang J P, Ji M X, Liu Y Z, et al. An overview of arid and semi-arid climate change[J]. Progressus Inquisitiones de Mutatione Climatis, 2013, 9(1): 9−14.
[2] Evenari M, Leslie S, Naphtali T. The negev: the challenge of a desert[M]. Boston: Harvard University Press, 1982.
[3] 王莉, 秦树高, 张宇清, 等. 生物土壤结皮对毛乌素沙地油蒿群落土壤水分的影响[J]. 北京林业大学学报, 2017, 39(3):48−56. Wang L, Qin S G, Zhang Y Q, et al. Influence of biological soil crusts on soil moisture in Artemisia ordosica community in Mu Us Desert[J]. Journal of Beijing Forestry University, 2017, 39(3): 48−56.
[4] 陈昌笃. 走向宏观生态学−陈昌笃论文集[M]. 北京: 科学出版社, 2009. Chen C D. To the macroscopic ecology-proceedings of Chen Chang-du[M]. Beijing: Science Press, 2009.
[5] 张军红. 毛乌素沙地油蒿群落生物结皮的分布特征[J]. 水土保持通报, 2014, 34(3):227−230. Zhang J H. Distribution characteristics of biological soil crust for Artemisia ordosica community in Mu Us Sandy Land[J]. Bulletin of Soil and Water Conservation, 2014, 34(3): 227−230.
[6] Weber B, Büdel B, Belnap J. Biological soil crusts: an organizing principle in drylands[M]. Berlin: Springer, 2016.
[7] Belnap J. The world at your feet: desert biological soil crusts[J]. Frontiers in Ecology and the Environment, 2003, 1(3): 181–189.
[8] 周健民, 沈仁芳. 土壤学大辞典[M]. 北京: 科学出版社, 2013. Zhou J M, Shen R F. Dictionary of soil science[M]. Beijing: Science Press, 2013.
[9] 鲍勇, 高颖, 曾晓敏, 等. 中亚热带3种典型森林土壤碳氮含量和酶活性的关系[J]. 植物生态学报, 2018, 42(4):508−516. Bao Y, Gao Y, Zeng X M, et al. Relationships between carbon and nitrogen contents and enzyme activities in soil of three typical subtropical forests in China[J]. Chinese Journal of Plant Ecology, 2018, 42(4): 508−516.
[10] 刘存歧, 王伟伟, 李贺鹏, 等. 湿地生态系统中土壤酶的研究进展[J]. 河北大学学报(自然科学版), 2005, 25(4):443−448. Liu C Q, Wang W W, Li H P, et al. Recent progress in studies on soil enzymes in wetland ecosystem[J]. Journal of Hebei University (Natural Science Edition), 2005, 25(4): 443−448.
[11] 向泽宇, 王长庭, 宋文彪, 等. 草地生态系统土壤酶活性研究进展[J]. 草业科学, 2011, 28(10):1801−1806. Xiang Z Y, Wang C T, Song W B, et al. Advances on soil enzymatic activities in grassland ecosystem[J]. Pratacultural Science, 2011, 28(10): 1801−1806.
[12] 于德良, 雷泽勇, 赵国军. 土壤酶活性对沙地樟子松人工林衰退的响应[J]. 环境化学, 2019, 38(1):1−9. Yu D L, Lei Z Y, Zhao G J. Response of soil enzyme activity to the decline of Pinus syluestris var. mongolica plantations on sand land[J]. Environmental Chemistry, 2019, 38(1): 1−9.
[13] 张体彬, 展小云, 冯浩. 盐碱地土壤酶活性研究进展和展望[J]. 土壤通报, 2017, 48(2):495−500. Zhang T B, Zhan X Y, Feng H. Research advance and prospect of soil enzymes activities in salinealkali soils[J]. Chinese Journal of Soil Science, 2017, 48(2): 495−500.
[14] 玛伊努尔·依克木, 张丙昌, 买买提明·苏来曼. 古尔班通古特沙漠生物结皮中微生物量与土壤酶活性的季节变化[J]. 中国沙漠, 2013, 33(4):1091−1097. Yikim Maynur, Zhang B C, Sulayman M. Seasonal variations of microbial biomass and soil enzyme activity in biological soil crusts in the Gurbantunggut Desert[J]. Journal of Desert Research, 2013, 33(4): 1091−1097.
[15] 杨航宇, 刘艳梅, 王廷璞. 荒漠区生物土壤结皮对土壤酶活性的影响[J]. 土壤学报, 2015, 52(3):654−664. Yang H Y, Liu Y M, Wang T P. Effects of biological soil crusts on soil enzyme activities in desert area[J]. Acta Pedologica Sinica, 2015, 52(3): 654−664.
[16] 唐春梅, 程胜高, 谢作明. 土壤藻改良退化草地对土壤酶活性的影响[J]. 环境科学与技术, 2018, 41(2):20−25. Tang C M, Cheng S G, Xie Z M. Effects of soil algae in degenerated grassland improvement on soil enzyme activities[J]. Environmental Science and Technology, 2018, 41(2): 20−25.
[17] Dettweiler-Robinson E, Sinsabaugh R L, Rudgers J. Biocrusts benefit from plant removal[J]. American Journal of Botany, 2018, 105: 1−9. doi: 10.1002/ajb2.1015.
[18] Sun Y, Zhang Y, Feng W, et al. Effects of xeric shrubs on soil microbial communities in a desert in northern China[J]. Plant and Soil, 2017, 414(1−2): 281−294. doi: 10.1007/s11104-016-3111-y.
[19] Delgado-Baquerizo M, Oliverio A M, Brewer T E, et al. A global atlas of the dominant bacteria found in soil[J]. Science, 2018, 359: 320−325. doi: 10.1126/science.aap9516.
[20] Porras-Alfaro A, Bayman P. Hidden fungi, emergent properties: endophytes and microbiomes[J]. Annual Review of Phytopathology, 2011, 49: 291−315. doi: 10.1146/annurev-phyto-080508-081831.
[21] Allen M F. Mycorrhizal fungi: highways for water and nutrients in arid soils[J]. Vadose Zone Journal, 2007, 6(2): 291−297. doi: 10.2136/vzj2006.0068
[22] Rudgers J A, Dettweiler-Robinson E, Belnap J, et al. Are fungal networks key to dryland primary production?[J]. American Journal of Botany, 2018, 105(11): 1783−1787. doi: 10.1002/ajb2.1184.
[23] Dettweiler-Robinson E. Biocrust carbon isotope signature was depleted under a C3 forb compared to interspace[J]. Plant and Soil, 2018, 429(1−2): 1−11. doi: 10.1007/s11104-018-3735-1.
[24] 雷雅凯. 毛乌素沙地油蒿种群格局研究[D]. 北京: 中国林业科学研究院, 2012. Lei Y K. Spatial pattern of Artemisia ordosica population in Mu Us Sandland, Inner Mongolia[D]. Beijing: Chinese Academy of Forestry, 2012.
[25] 冯薇. 毛乌素沙地生物结皮光合固碳过程及对土壤碳排放的影响[D]. 北京: 北京林业大学, 2014. Feng W. Photosythetic carbon fixation of biological soil crusts in Mu Us Desert and their impact on soil carbon emission[D]. Beijing: Beijing Forestry University, 2014.
[26] Černohlávková J, Jarkovský J, Nesporová M, et al. Variability of soil microbial properties: effects of sampling, handling and storage[J]. Ecotoxicology and Environmental Safety, 2009, 72(8): 2100−2108.
[27] 关松荫. 土壤酶及其研究法[M]. 北京: 农业出版社, 1983. Guan S Y. Soil enzyme and its research method[M]. Beijing: China Agriculture Press, 1983.
[28] 陈青. 荒漠生物结皮微生物群落组成研究[D]. 银川: 宁夏大学, 2014. Chen Q. Investigation of microbial communities structure in biological soil crusts of desert[D]. Yinchuan: Ningxia University, 2014.
[29] Porras-Alfaro A, Herrera J, Sinsabaugh R L, et al. Novel root fungal consortium associated with a dominant desert grass[J]. Applied and Environmental Microbiology, 2008, 74(9): 2805−2813. doi: 10.1128/AEM.02769-07.
[30] Fang C, Smith P, Smith J U, et al. Incorporating microorganisms as decomposers into models to simulate soil organic matter decomposition[J]. Geoderma, 2016, 129(3−4): 139−146. doi: 10.1016/j.geoderma.2004.12.038.
[31] Steven B, Yeager C, Belnap J, et al. Common and distinguishing features of the bacterial and fungal communities in biological soil crusts and shrub root zone soils[J]. Soil Biology and Biochemistry, 2014, 69: 302−312. doi: 10.1016/j.soilbio.2013.11.008.
[32] Zhang B, Kong W, Wu N, et al. Bacterial diversity and community along the succession of biological soil crusts in the Gurbantunggut Desert, Northern China[J]. Journal of Basic Microbiology, 2016, 56(6): 670−679. doi: 10.1002/jobm.201500751.
[33] Liu L, Liu Y, Hui R, et al. Recovery of microbial community structure of biological soil crusts in successional stages of Shapotou Desert revegetation, northwest China[J]. Soil Biology and Biochemistry, 2017, 107: 125−128. doi: 10.1016/j.soilbio.2016.12.030.
[34] Fu S, Cheng W. Defoliation affects rhizosphere respiration and rhizosphere priming effect on decomposition of soil organic matter under a sunflower species: Helianthus annuus[J]. Plant and Soil, 2004, 263(1): 345−352. doi: 10.1023/B:PLSO.0000047745.30929.32
[35] Xiao B, Veste M. Moss-dominated biocrusts increase soil microbial abundance and community diversity and improve soil fertility in semi-arid climates on the Loess Plateau of China[J]. Applied Soil Ecology, 2017, 117−118: 165−177. doi: 10.1016/j.apsoil.2017.05.005.
[36] Zaady E, Ben-David E A, Sher Y, et al. Inferring biological soil crust successional stage using combined PLFA, DGGE, physical and biophysiological analyses[J]. Soil Biology and Biochemistry, 2010, 42(5): 842−849. doi: 10.1016/j.soilbio.2010.02.002.
[37] Vitousek P M, Porder S, Houlton B Z, et al. Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen-phosphorus interactions[J]. Ecological Applications, 2010, 20(1): 5−15. doi: 10.1890/08-0127.1.
[38] Bates S T, Cropsey G W, Caporaso J G, et al. Bacterial communities associated with the lichen symbiosis[J]. Applied and Environmental Microbiology, 2011, 77(4): 1309−1314. doi: 10.1128/AEM.02257-10
[39] Maier S, Schmidt T S, Zheng L, et al. Analyses of dryland biological soil crusts highlight lichens as an important regulator of microbial communities[J]. Biodiversity and Conservation, 2014, 23(7): 1735−1755. doi: 10.1007/s10531-014-0719-1.
[40] Burns R G, Deforest J L, Marxsen J, et al. Soil enzymes in a changing environment: current knowledge and future directions[J]. Soil Biology and Biochemistry, 2013, 58: 216−234. doi: 10.1016/j.soilbio.2012.11.009.
[41] Johnson S L, Budinoff C R, Belnap J, et al. Relevance of ammonium oxidation within biological soil crust communities[J]. Environmental Microbiology, 2005, 7(1): 1−12. doi: 10.1111/j.1462-2920.2004.00649.x.
[42] Barger N N. Biogeochemical cycling and N dynamics of biological soil crusts in semi-arid ecosystem[M]. Fort Collins: Colorado State University, 2003.
[43] She W, Bai Y, Zhang Y, et al. Resource availability drives responses of soil microbial communities to short-term precipitation and nitrogen addition in a desert shrubland[J/OL]. Frontiers in Microbiology, 2018, 9: 186 [2019−05−11]. https://doi.org/10.3389/fmicb.2018.00186.
[44] 张国秀, 赵允格, 许明祥, 等. 黄土丘陵区生物结皮对土壤磷素有效性及碱性磷酸酶活性的影响[J]. 植物营养与肥料学报, 2012, 18(3):621−628. Zhang G X, Zhao Y G, Xu M X, et al. Impacts of biological soil crust on availability of phosphorus and phosphatase activity in hilly regions of the Loess Plateau, China[J]. Journal of Plant Nutrition and Fertilizers, 2012, 18(3): 621−628.
[45] 陆文龙, 曹一平, 张福锁. 根分泌的有机酸对土壤磷和微量元素的活化作用[J]. 应用生态学报, 1999, 10(3):124−127. Lu W L, Cao Y P, Zhang F S. Role of root-exuded organic acids in mobilization of soil phosphorus and micronutrients[J]. Chinese Journal of Applied Ecology, 1999, 10(3): 124−127.
[46] Steven B, Gallegos-Graves L V, Belnap J, et al. Dryland soil microbial communities display spatial biogeographic patterns associated with soil depth and soil parent material[J]. FEMS Microbiology Ecology, 2013, 86(1): 101−113. doi: 10.1111/1574-6941.12143.
[47] 闫德仁, 黄海广, 张胜男, 等. 沙漠苔藓生物结皮层养分及颗粒组成特征[J]. 干旱区资源与环境, 2018, 32(10):111−116. Yan D R, Huang H G, Zhang S N, et al. Nutrients and particle composition characteristics in moss biological crusts[J]. Journal of Arid Land Resources and Environment, 2018, 32(10): 111−116.
-
期刊类型引用(12)
1. 孙凡,马彦广,刘占民,杨博宁,王辉丽,李伟. 油松高世代种子园亲本选择策略研究. 北京林业大学学报. 2024(04): 28-39 .  本站查看
本站查看
2. 吕寻,李万峰,胡勐鸿,戴小芬,成红梅,委霞. 日本落叶松种子园和优树自由授粉家系选择与利用研究. 西南林业大学学报(自然科学). 2024(03): 1-9 .  百度学术
百度学术
3. 冯健,张金博,杨圆圆,杜超群,徐柏松,曹颖,姚飞. 基于生长性状和SSR遗传多样性分析的红松第二代优树选择研究. 西南林业大学学报(自然科学). 2024(04): 1-7 .  百度学术
百度学术
4. 胡勐鸿,吕寻,戴小芬,李宗德,李万峰. 日本落叶松无性系种子园和优树半同胞家系苗期比较. 东北林业大学学报. 2024(12): 10-17 .  百度学术
百度学术
5. 向华,向文明,向明. 我国用材林优树选择技术研究进展. 湖南林业科技. 2021(02): 89-96 .  百度学术
百度学术
6. 康向阳. 林木遗传育种研究进展. 南京林业大学学报(自然科学版). 2020(03): 1-10 .  百度学术
百度学术
7. 邓乐平,黄婷,王哲,吴惠姗,李晓华,廖仿炎,李义良,郭文冰,赵奋成. 湿地松改良种子园无性系的遗传评价及新一轮育种亲本选择. 林业与环境科学. 2020(04): 1-7 .  百度学术
百度学术
8. 杜超群,赵虎,袁慧,侯义梅,朱于勤,许业洲. 日本落叶松种子园母树生长及种实性状评价. 森林与环境学报. 2019(01): 32-36 .  百度学术
百度学术
9. 王芳,王元兴,王成录,张伟娜,刘卫胜,陆志民,杨雨春. 红松优树半同胞子代家系生长、结实及抗病虫能力的变异特征. 应用生态学报. 2019(05): 1679-1686 .  百度学术
百度学术
10. 金星,于忠峰,苗海伟,于国斌,朱瑞,张丽杰. 辽宁地区油松花粉形态及生活力的测定. 分子植物育种. 2019(15): 5115-5119 .  百度学术
百度学术
11. 康向阳. 关于林木育种策略的思考. 北京林业大学学报. 2019(12): 15-22 .  本站查看
本站查看
12. 苗禹博,朱晓梅,李志娟,贾凤岭,李伟. 不同世代樟子松育种资源遗传评价. 北京林业大学学报. 2017(12): 71-78 .  本站查看
本站查看
其他类型引用(4)



 下载:
下载:

