Anatomical structure characteristics and growth ring analysis of underground rhizome of herbaceous peony
-
摘要:目的观察分析不同芍药品种群品种的根茎结构发育特点,对芍药根茎进行解剖学研究,初步解析芍药根茎次生结构及生长轮特征,以期为通过根茎结构判断芍药株龄、优化无性繁殖和栽培管理技术,促进资源的开发和利用研究提供一定的理论依据。方法以芍药不同品种群的6个品种不同发育时期的地下根茎为研究对象,观察并分析其发育更新特征,应用石蜡切片和徒手切片技术,对不同品种、同一品种不同生长年限的根茎次生结构特征进行观察分析。结果不同芍药品种的地下根茎组织架构基本一致,即由根茎、着生于根茎上的根茎芽和根3部分组成,根茎每年有序更新,年龄分级特征明显;四倍体品种‘Cream Delight’根茎发育特征与其余5个品种差异明显;6个芍药品种根茎次生结构由周皮、皮层、次生韧皮部、形成层、次生木质部和中央髓组成。二倍体‘种生粉’、二倍体‘粉玉奴’、三倍体‘Coral Sunset’和四倍体‘Cream Delight’4个品种根茎次生木质部大小导管有规律地依次排列,口径较大的导管和周围的小导管聚集形成群团状,导管群分布较稀疏,两导管群之间的间隔明显。与四倍体‘Cream Delight’相比,三倍体‘Coral Sunset’的导管群分布较紧凑。伊藤杂种两个三倍体品种‘Prairie Charm’和‘Going Bananas’根茎的次生木质部大小导管分布较均匀,形成较连续的环带,并不聚集形成团块状。芍药根茎截面在脱水后维管组织呈白色或淡黄色,间断环状分布,中央髓部组织下凹,位于不同环的维管组织从髓部向皮层呈放射状排列。不同生长年限的根茎中次生木质部口径较大的导管及其周围的小导管聚集呈团块状,导管群切向断续有规律地依次排列成与形成层平行的环,形成清晰的生长轮,且生长轮的数目与其实际生长年龄一致。结论芍药不同品种地下根茎组织架构特征基本一致,且存在明显的龄级特征。二倍体及三倍体各品种根茎发育特征相似,而与四倍体不同。中国芍药及杂种芍药品种群品种根茎次生结构相似而与伊藤杂种差异明显,杂种芍药品种群内三倍体及四倍体品种根茎次生结构差异较大,芍药根茎生长轮次生结构特征与品种倍性无关。芍药根茎中的生长轮即为其年轮,且其数目能够反映芍药的实际生长年限。Abstract:ObjectiveBy observing and analyzing the rhizome structure and development characteristics of different varieties of peony, the anatomical study on the rhizome was conducted to preliminarily analyze the secondary structure and growth ring characteristics of the rhizome of herbaceous peony, so as to provide theoretical basis for judging the plant age of peony and optimizing the asexual reproduction and cultivation management technology, and promote the development and utilization of resources.MethodThe underground rhizomes of different developmental stages of six varieties of different peony groups were studied, and their development and renewal characteristics were observed and analyzed. Paraffin sections and freehand slicing techniques were used to observe and analyze the secondary structural characteristics of rhizomes of different cultivars and different cultivars with varied growth years.ResultThe underground rhizome structure of different varieties was basically the same, that is, the underground organs of the peony were composed of rhizome, rhizome buds and roots growing on the rhizome. The rhizome was updated regularly every year, and the age grading characteristics were obvious; the developmental characteristics of the tetraploid cultivar ‘Cream Delight’ were significantly different from the other five varieties.The rhizomes of 6 varieties of peony were composed of peridermis, cortex, secondary phloem, cambium, secondary xylem and central pith. The large and small conduits of the secondary xylem of the rhizomes of diploid variety ‘Zhongshengfen’, diploid variety ‘Fenyunu’, triploid variety ‘Coral Sunset’ and tetraploid variety ‘Cream Delight’ were arranged sequentially.The larger diameter catheter and the surrounding small catheter gathered to form a cluster, and the catheter group was sparsely distributed. The spacing between the two catheter groups was significant. The interval between the catheter clusters of ‘Coral Sunset’ was smaller than ‘Cream Delight’.The large and small ducts in the secondary xylem of the rhizomes of the two triploid Itoh varieties ‘Prairie Charm’ and ‘Going Bananas’ were evenly distributed and formed a relatively continuous ring zone, and they don’t clump together. After dehydration, the vascular structure of the rhizome of the peony was white or yellowish, with intermittent distribution of the ring, and the central medullary tissue was concave. Vascular tissues located in different rings were radially arranged from the pith to the cortex. The larger diameter catheters in the secondary xylem and the small ones around them were clustered in clumps in the rhizomes of different growth years. The catheter group was tangentially intermittently arranged in a sequence parallel to the cambium to form a clear growth ring, and the number of growth ring was consistent with the actual growth age of the rhizome of the herbaceous peony.ConclusionThe structural characteristics of underground rhizome of different varieties of peony are basically the same, and there are obvious grading characteristics. The rhizome development characteristics of diploid and triploid are similar, but different from tetraploid. The secondary structural characteristics of rhizome of varieties belonging to lactiflora and hybrid groups are similar, but are significantly different in the varieties of Itoh group. There are significant differences in secondary structure of the rhizomes between triploid and tetraploid varieties of hybrid groups, and there is no direct correlation between the secondary structure characteristics of growth ring of rhizome and chromosome ploidy of herbaceous peony. The growth rings in the rhizome of herbaceous peony are its annual rings, and the number of growth rings can reflect the actual growth years of herbaceous peony.
-
Keywords:
- herbaceous peony /
- underground rhizome /
- growth ring /
- growth year /
- anatomical characteristics
-
植物的寿命是其生活史上一个重要的特征,准确判断植物的生长年龄对理解植物在特定环境中的发育和繁殖更新状况,评估其生长影响因子及环境适应能力,由此制定合理的栽培管理及开发利用措施意义重大[1-5]。植物生长年龄一般可以通过周年生长形态、物候及生长轮等进行分析。在木本植物中,多年生茎中的年轮解剖结构特征可以反映其实际生长年限及生长发育状况[1,6]。研究表明,多年生草本植物位于地下的宿存器官(根茎,块茎,块根和鳞茎)中存在类似树木年轮的“生长轮”可以作为其生长年限判别的依据[1]。根茎是根茎类植物营养物质的重要贮存场所[7-9],也是其自然更新和分株繁殖的主要器官,是芽与根生理整合的枢纽通道[10],对根茎的形态特征、次生结构及其生长年龄的研究是揭示其生长发育及环境适应机制的重要基础,因而受到广泛关注。目前,对根茎生长年龄的判断一方面是根据其世代繁殖更新的形态特征来确定[2-5],另一方面可以通过其生长轮来进行判断,在灰白千里光(Senecio incanus),草甸鼠尾草(Salvia pratensis )及奇异蜂斗菜(Petasites paradoxus)等植物的根茎中均发现有生长轮的存在[1]。
芍药(herbaceous peony)是典型的根茎类多年生草本植物,野生遗传资源丰富[11],栽培品种繁多,形成了3大品种类群[12-15],有重要的观赏和药用价值[16-17]。目前关于芍药根茎的研究报道较少,仅对中国芍药品种群的个别品种的根茎生长发育及初生组织结构特征进行了初步的研究[18],对芍药根茎中是否存在生长轮以及不同品种间的生长轮差异尚未有报道。因此,本研究通过比较观察分析不同芍药品种群品种的根茎形态生长发育特点,对芍药根茎进行解剖学研究,观察其生长轮特点,判别其生长年限,以期为芍药合理栽培措施的制定、无性繁殖技术的优化及资源的开发利用研究提供一定的基础理论指导。研究结果对其他多年生根茎类植物的生长年龄判断及相关研究的开展也具有一定的借鉴意义。
1. 材料与方法
1.1 材 料
芍药不同品种群品种(表1),种植于国家花卉工程中心芍药种质资源圃(北京昌平区小汤山镇),供试材料为3年生分株苗。
表 1 供试芍药品种信息Table 1. Variety information of experimental materials编号 No. 品种名称 Variety name 品种群分类 Classification of cultivar groups 倍性 Ploidy level 1 ‘种生粉’ ‘Zhongshengfen’ 中国芍药品种群 Lactiflora group 2n = 2x = 10 2 ‘粉玉奴’ ‘Fenyunu’ 中国芍药品种群 Lactiflora group 2n = 2x = 10 3 ‘珊瑚落日’ ‘Coral Sunset’ 杂种芍药品种群 Hybrid group 2n = 3x = 15 4 ‘乳霜之愉’ ‘Cream Delight’ 杂种芍药品种群 Hybrid group 2n = 4x = 20 5 ‘草原风情’ ‘Prairie Charm’ 伊藤杂种品种群 Itoh hybrid group 2n = 3x = 15 6 ‘抓狂的香蕉’ ‘Going Bananas’ 伊藤杂种品种群 Itoh hybrid group 2n = 3x = 15 1.2 方 法
1.2.1 芍药地下根茎发育更新特点观察
于2018年9月中旬至11月底,剪除芍药地上枯茎,将地下根茎整体起挖,去除泥土、杂物,沿根茎生长方向将其理顺并去除多余的肉质根以使根茎清晰可见,拍照观察并记录芍药根茎的生长更新特征。
1.2.2 芍药不同品种地下根茎解剖结构观察
选取不同品种当年生根茎芽基部1 cm以下的成熟根茎组织,沿其横轴切取约2 mm的薄片,FAA固定液(50%乙醇∶甲醛∶冰醋酸 = 90∶5∶5,体积比)真空固定处理24 h以上,随后加入约1/5 FAA体积甘油软化处理10 d以上,随后经脱水,透明,浸蜡,包埋后切片,切片厚度16 ~ 25 μm,经固绿−番红染色后中性树胶封片,Leica EZ4HD体式显微镜观察和拍照。
1.2.3 芍药根茎生长轮观察
体式显微观察:按照根茎的着生规律,切取发育正常的不同生长年限的根茎,冰盒保存带至实验室,用自来水冲洗3遍,去除根茎表面的泥土及其他杂质,置于吸水纸上室温晾1 ~ 2 h左右,观察时不锈钢刀片沿其横轴方向截平,将切口晾3 ~ 5 min后Leica EZ4HD体式显微镜拍照观察。
石蜡切片制备:按照根茎的着生规律,选取发育正常的芍药不同生长年限的根茎按1.2.2所述方法进行切片观察。
2. 结果与分析
2.1 芍药地下根茎结构发育特点
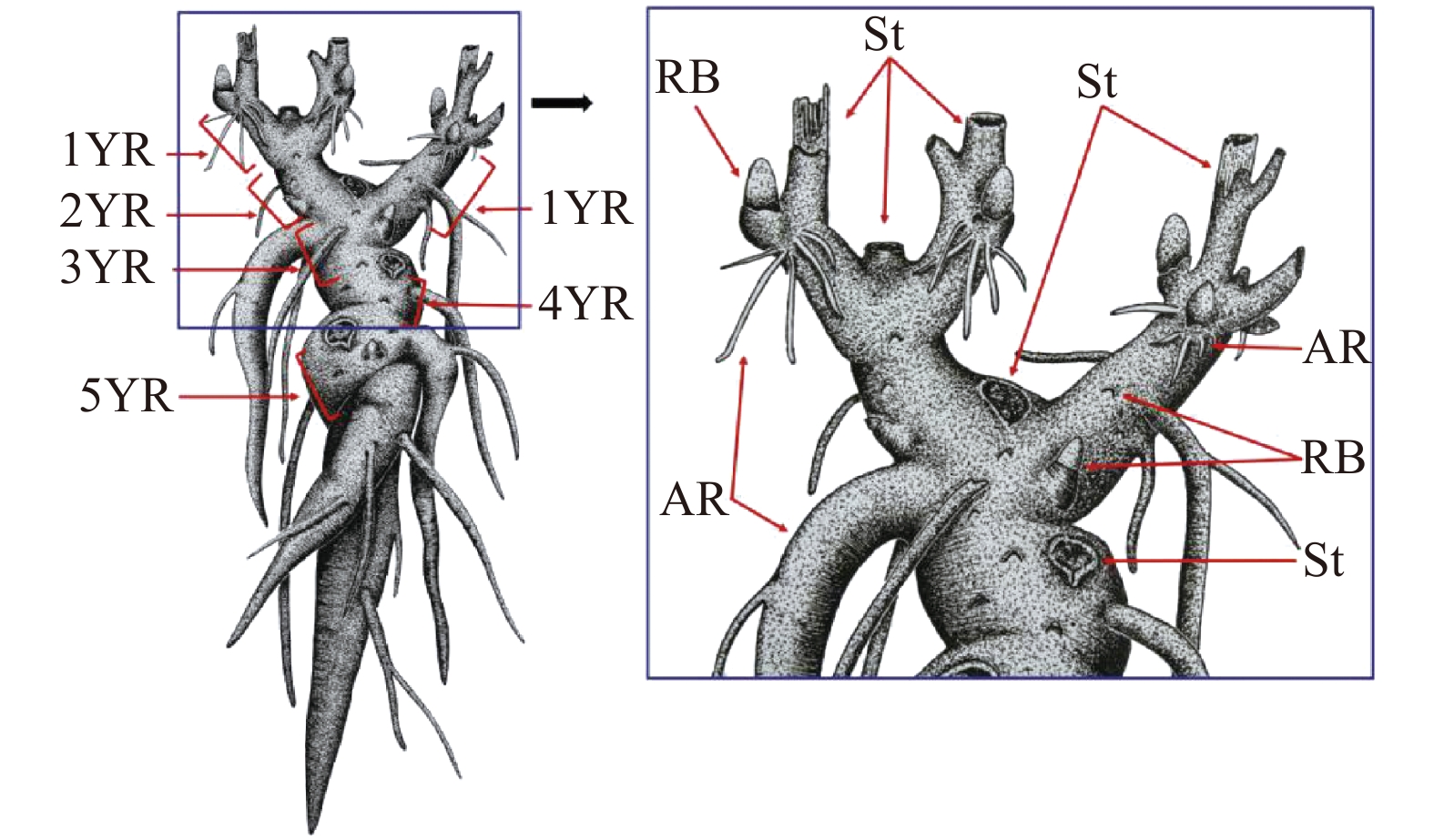
不同芍药品种群品种植株地下的组织架构基本一致,即由根茎、着生于根茎上的根茎芽和根3部分组成,根茎与肉质根在颜色上基本一致,除顶部根茎上着生的根茎芽外,下部根茎上也宿存大量处于休眠状态的根茎芽。正常发育的芍药地下根茎发育形态具有较明显的年龄分级特征,我们把当年根茎芽萌发后形成的根茎作为1龄生根茎,则1龄生根茎所着生的上一年形成的母代根茎则为2龄生根茎,2龄生根茎所着生的上一年形成的母代根茎为3龄生根茎,以此类推,各生长年限的根茎之间以根茎上宿存的茎或者残留的茎痕为界,偶见有当年生根茎着生于2龄以上的母代根茎(图1)。
![]() 图 1 芍药根茎结构发育示意1YR:1龄生根茎;2YR:2龄生根茎;3YR:3龄生根茎;4YR:4龄生根茎;5YR:5龄生根茎;RB:根茎芽;St:茎;AR:不定根。1YR, 1 year old rhizome; 2YR, 2 years old rhizome; 3YR, 3 years old rhizome; 4YR, 4 years old rhizome; 5YR, 5 years old rhizome; RB, rhizome bud; St, stem; AR, adventitious root.Figure 1. Structural characteristic of rhizome of herbaceous peony
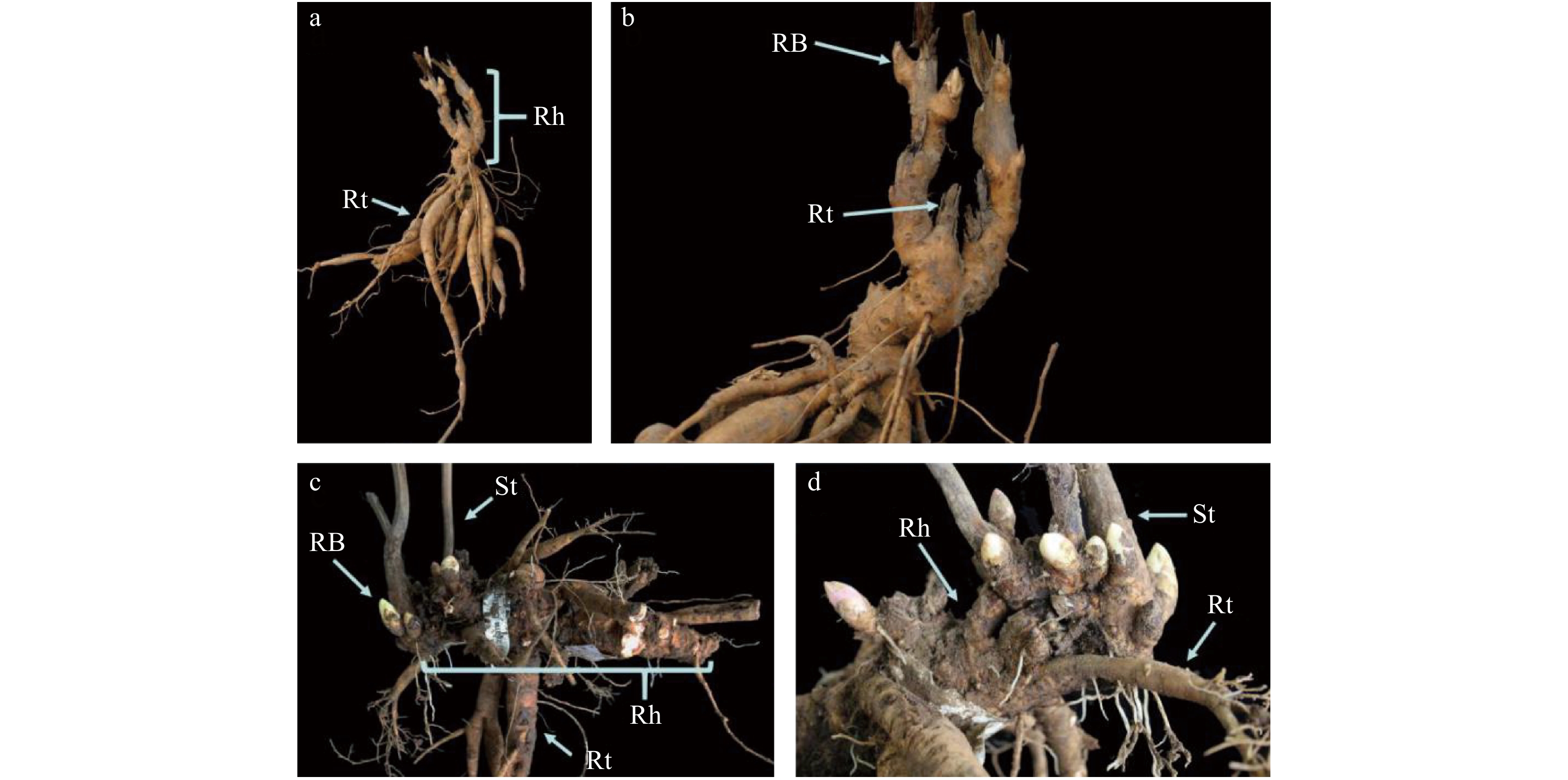
图 1 芍药根茎结构发育示意1YR:1龄生根茎;2YR:2龄生根茎;3YR:3龄生根茎;4YR:4龄生根茎;5YR:5龄生根茎;RB:根茎芽;St:茎;AR:不定根。1YR, 1 year old rhizome; 2YR, 2 years old rhizome; 3YR, 3 years old rhizome; 4YR, 4 years old rhizome; 5YR, 5 years old rhizome; RB, rhizome bud; St, stem; AR, adventitious root.Figure 1. Structural characteristic of rhizome of herbaceous peony‘种生粉’‘粉玉奴’‘Coral Sunset’‘Prairie Charm’和‘Going Bananas’5个品种地下根茎结构形态类似:每年的纵向(长度)生长量适中且横向(直径)膨大变异较小,不同龄级的根茎组织结构易于区分,且根茎背地向上更新(图2a、b)。四倍体品种‘Cream Delight’每年纵向生长量小而横向生长量较大,膨大明显,不同龄级的根茎组织结构紧凑而不易区分,且往往与地表水平方向横向更新(图2c、d)。
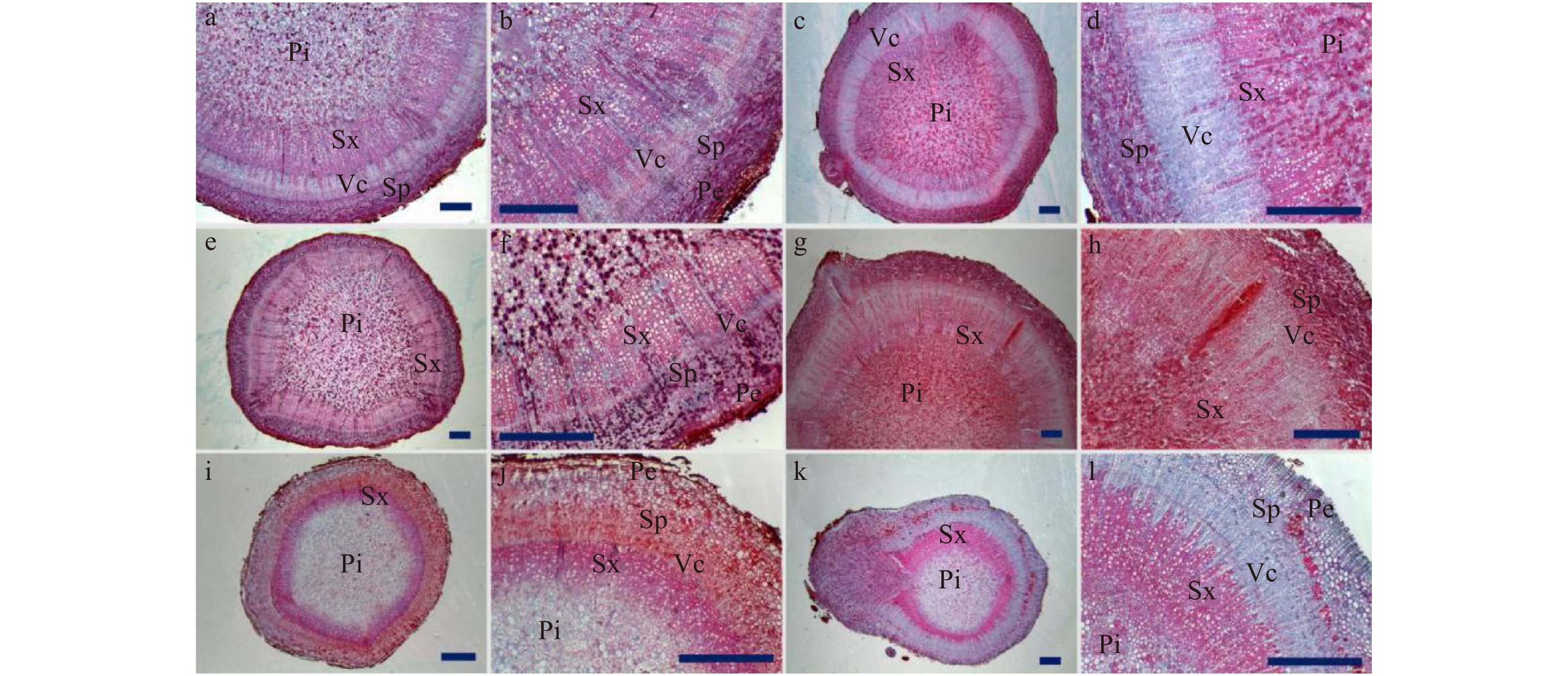
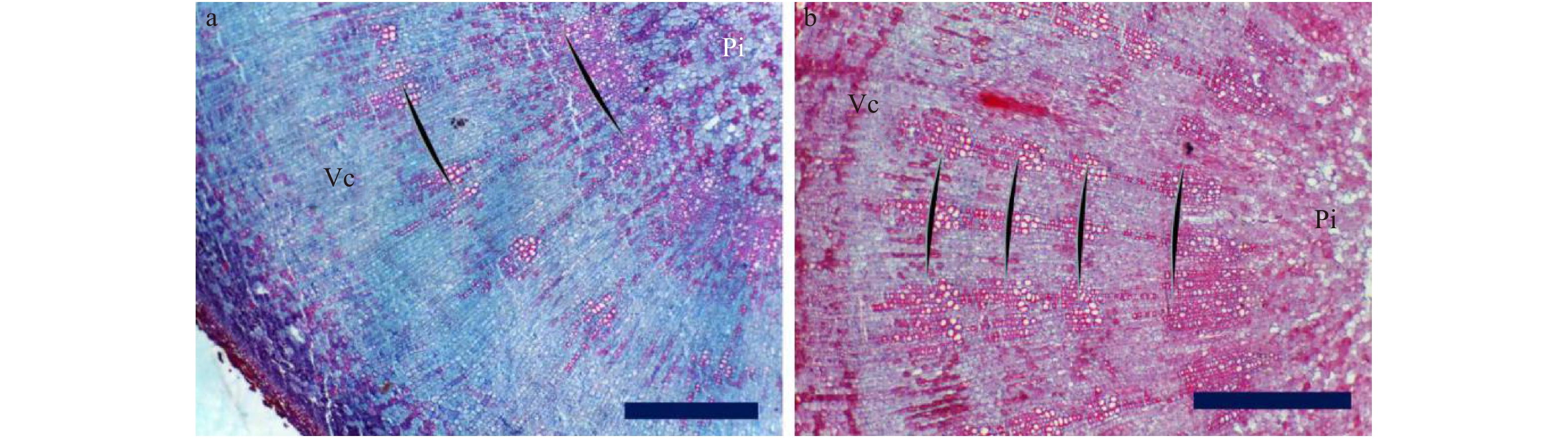
6个芍药品种根茎截面解剖构造均符合双子叶植物茎的次生构造,由周皮、皮层、次生韧皮部、形成层、次生木质部和中央髓组成(图3)。
![]() 图 3 不同品种根茎解剖结构a、b. ‘大富贵’根茎解剖结构;c、d. ‘粉玉奴’根茎解剖结构;e、f. ‘珊瑚落日’根茎解剖结构;g、h. ‘乳霜之愉’根茎解剖结构;i、j. ‘草原风情’根茎解剖结构;k、l. ‘抓狂的香蕉’根茎解剖结构;Pe:周皮;Sp:次生韧皮部;Vc:维管形成层;Sx:次生木质部;Pi:髓。标尺 = 1 000 μm。a, b, rhizome anatomy of ‘Dafugui’; c, d, rhizome anatomy of ‘Fenyunu’; e, f, rhizome anatomy of ‘Coral Sunset’; g, h, rhizome anatomy of ‘Cream Delight’; i, j, rhizome anatomy of ‘Prairie Charm’; k, l, rhizome anatomy of ‘Going Bananas’; Pe, periderm; Sp, secondary phloem; Vc, vascular cambium; Sx, secondary xylem; Pi, pith. Scale bar = 1 000 μm.Figure 3. Anatomical structure of rhizomes of different cultivars of herbaceous peony
图 3 不同品种根茎解剖结构a、b. ‘大富贵’根茎解剖结构;c、d. ‘粉玉奴’根茎解剖结构;e、f. ‘珊瑚落日’根茎解剖结构;g、h. ‘乳霜之愉’根茎解剖结构;i、j. ‘草原风情’根茎解剖结构;k、l. ‘抓狂的香蕉’根茎解剖结构;Pe:周皮;Sp:次生韧皮部;Vc:维管形成层;Sx:次生木质部;Pi:髓。标尺 = 1 000 μm。a, b, rhizome anatomy of ‘Dafugui’; c, d, rhizome anatomy of ‘Fenyunu’; e, f, rhizome anatomy of ‘Coral Sunset’; g, h, rhizome anatomy of ‘Cream Delight’; i, j, rhizome anatomy of ‘Prairie Charm’; k, l, rhizome anatomy of ‘Going Bananas’; Pe, periderm; Sp, secondary phloem; Vc, vascular cambium; Sx, secondary xylem; Pi, pith. Scale bar = 1 000 μm.Figure 3. Anatomical structure of rhizomes of different cultivars of herbaceous peony‘种生粉’‘粉玉奴’‘Coral Sunset’和‘Cream Delight’4个品种根茎次生木质部显微结构类似:大小导管有规律地依次排列,口径较大的导管和周围的小导管聚集形成群团状,导管群分布较稀疏,两导管群之间的间隔明显。与‘Cream Delight’相比,‘Coral Sunset’的导管群分布较紧凑。‘Prairie Charm’和‘Going Bananas’根茎的次生木质部大小导管分布较均匀,形成较连续的环带,并不聚集形成团块状(图3)。
2.2 芍药不同生长年限根茎横切面特征
芍药根茎截面在脱水后维管组织凸起,呈白色或淡黄色,间断环状分布,中央髓部组织下凹,位于不同环的维管组织从髓部向皮层呈放射状排列(图4)。
次生木质部显微观察结果显示,口径较大的导管及其周围的小导管聚集呈团块状,导管群切向断续排列成与形成层平行的环,形成清晰的生长轮(图5)。
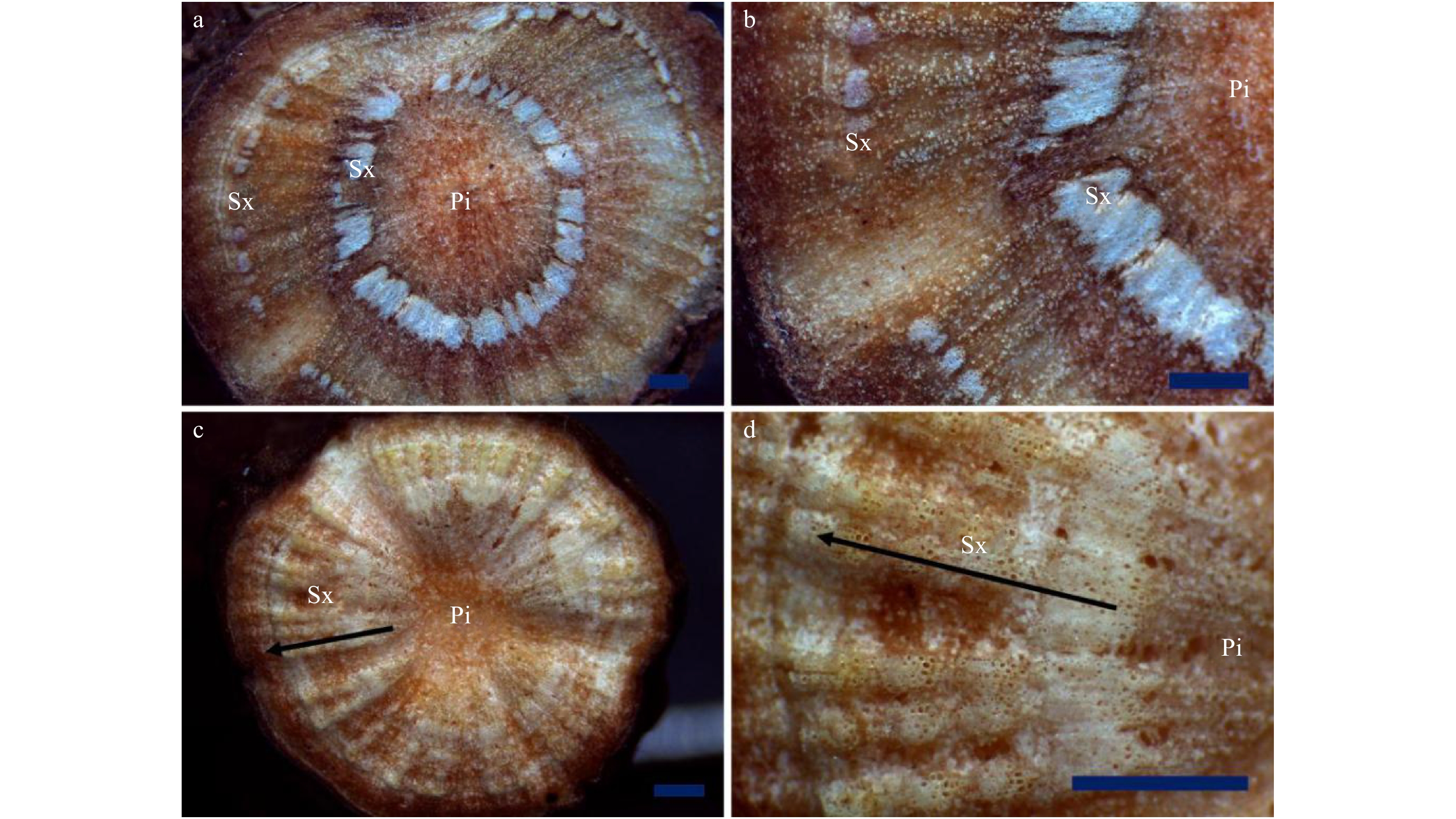
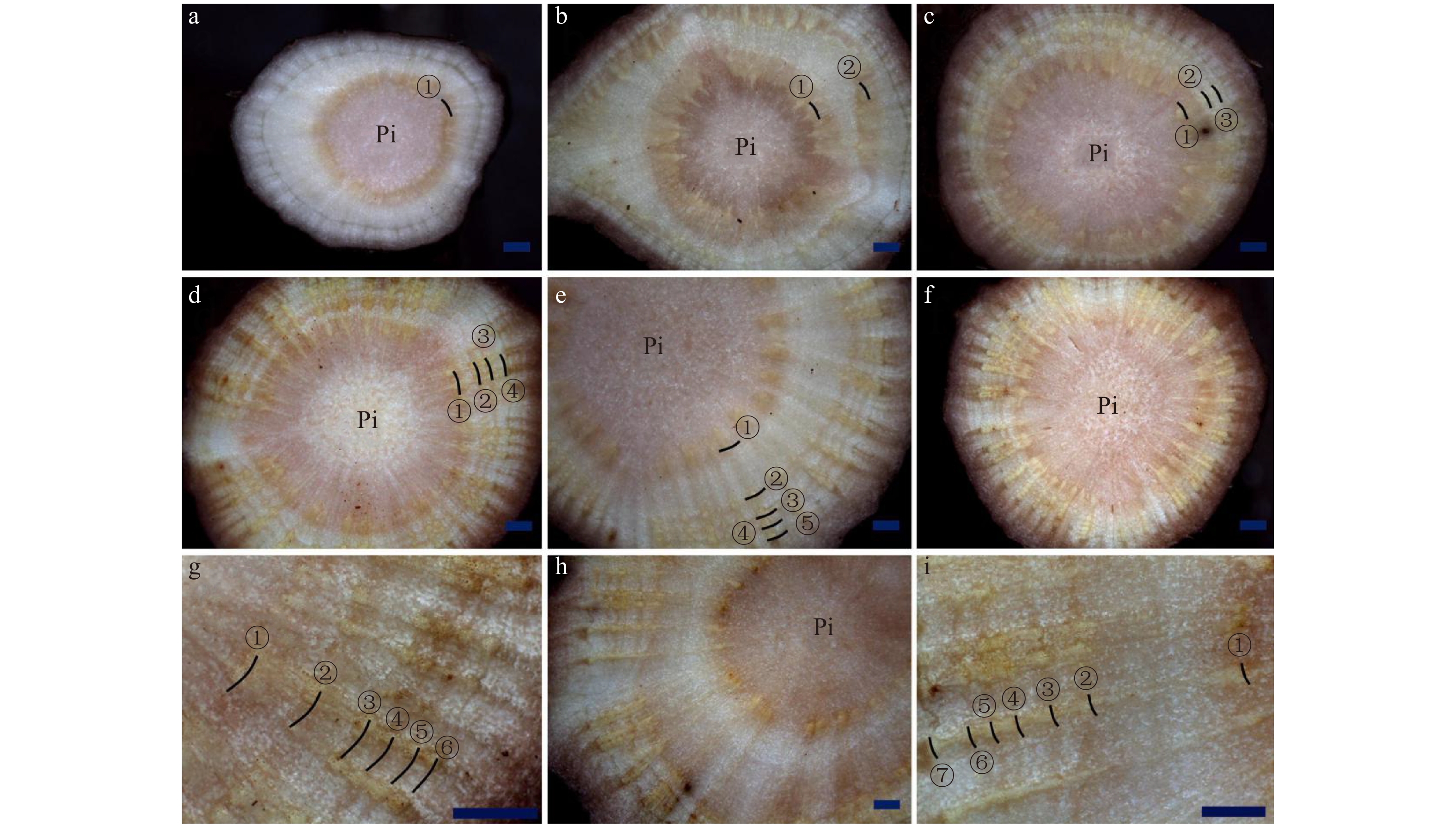
对生长发育正常的芍药不同生长年限根茎进行组织切片观察发现,一年生根茎生长轮数目为1(图6a),2年生根茎生长轮的数目为2(图6b),3年生根茎的生长轮数目为3(图6c),依此类推。生长轮的数目与其实际生长年限一致。
![]() 图 6 芍药根茎不同生长年限生长轮观察a. 1龄生根茎;b. 2龄生根茎;c. 3龄生根茎;d. 4龄生根茎;e. 5龄生根茎;f、g. 6龄生根茎;h、i. 7龄生根茎;①. 第1个生长轮;②. 第2个生长轮;③. 第3个生长轮;④. 第4个生长轮;⑤. 第5个生长轮;⑥. 第6个生长轮;⑦. 第7个生长轮。标尺 = 1 000 μm。a, 1 year old rhizome; b, 2 years old rhizome; c, 3 years old rhizome; d, 4 years old rhizome; e, 5 years old rhizome; f, g, 6 years old rhizome; h, i, 7 years old rhizome; ①, the first growth ring; ②, the second growth ring; ③, the third growth ring; ④, the fourth growth ring; ⑤, the fifth growth ring; ⑥, the sixth growth ring; ⑦, the seventh growth ring. Scale bar = 1 000 μm.Figure 6. Observation on the growth rings of the rhizome of herbaceous peony under a stereomicroscope
图 6 芍药根茎不同生长年限生长轮观察a. 1龄生根茎;b. 2龄生根茎;c. 3龄生根茎;d. 4龄生根茎;e. 5龄生根茎;f、g. 6龄生根茎;h、i. 7龄生根茎;①. 第1个生长轮;②. 第2个生长轮;③. 第3个生长轮;④. 第4个生长轮;⑤. 第5个生长轮;⑥. 第6个生长轮;⑦. 第7个生长轮。标尺 = 1 000 μm。a, 1 year old rhizome; b, 2 years old rhizome; c, 3 years old rhizome; d, 4 years old rhizome; e, 5 years old rhizome; f, g, 6 years old rhizome; h, i, 7 years old rhizome; ①, the first growth ring; ②, the second growth ring; ③, the third growth ring; ④, the fourth growth ring; ⑤, the fifth growth ring; ⑥, the sixth growth ring; ⑦, the seventh growth ring. Scale bar = 1 000 μm.Figure 6. Observation on the growth rings of the rhizome of herbaceous peony under a stereomicroscope3. 讨 论
根茎的形态及生长年限反映了植物在特定气候环境条件下的生长发育状况。准确判断根茎的年龄结构对预知植物个体乃至种群繁殖发育现状及未来更新的动态发展,由此制定合理的栽培及开发利用措施意义重大[1]。目前,对根茎类植物年龄结构的判断尚无统一标准和方法,一般是根据其实际栽培年限[8, 19-21]、营养繁殖世代特征结合颜色及直径大小等进行判断[2-3]。本研究中,芍药每年夏秋形成的当年生根茎由位于上一年形成的母代根茎芽发育而来,由此逐年进行世代更替,通过这种繁殖世代特征可以初步判断芍药根茎的年龄结构。芍药根茎芽的更新严格受控于顶端优势的调控[22],因而在发育正常的情况下,芍药的根茎一般按照实际生长年限逐级生长[18],但是,在本研究中,我们观察到在一些植株中当年生根茎由2龄或更高级年龄的母代根茎发育而来,若非经全株整体观察及长时间的持续追踪,完全按照根茎由上至下的分级次序来判别每一级根茎的生长年限往往存在一定的困难,对母代根茎的实际生长年限易造成误判。近年来兴起的草本植物生长轮研究为草本植物生长年限的研究提供了新的思路[1, 17, 21]。本研究中,芍药根茎的初生结构与茎的结构基本类似,由表皮、皮层、维管束和中央髓组成[18, 23]。与地上茎不同的是,芍药根茎的次生结构外围形成了具有保护作用的周皮组织,因而其能多年宿存生长。芍药不同龄级根茎中存在明显的生长轮,且生长轮的数目与其对应根茎的实际生长年限一致,可以作为判别芍药根茎实际生长年限的稳定依据。
芍药根茎生长轮的组织形态和根茎的发育状况受植物本身遗传差异和栽培环境的影响。本研究中,考虑到供试样本栽培环境基本一致,不同品种根茎形态及生长轮的差异可能主要与其亲本来源不同有关。中国芍药品种群和杂种芍药品种群各品种亲本来源于芍药属(Paeonia)的多年生草本植物类群,品种群内各品种根茎生长轮的组织结构类似,木质部导管群断续排列成环,而伊藤杂种品种群内两个品种根茎次生木质部呈现连续的环带分布,主要是由于其亲本融合了芍药属亚灌木的牡丹类群的遗传信息,因而生长轮结构与牡丹茎的次生结构类似[23],据此,可以将其与其他两个品种群的品种进行区分。至于这种次生结构差异对其存活年限的影响有待进一步研究。
植物多倍体往往具有营养器官大、抗逆性强、生长迅速等特点[24-27]。与二倍体品种相比,芍药多倍体品种往往也表现出茎秆粗壮、直立性强等生长优势[15,28-29]。本研究中,从根茎生长表现来看,四倍体品种‘Cream Delight’相同龄级的根茎体量明显大于二倍体及三倍体品种,由于根茎每年生长量大,加之向地伸展空间有限,因而多呈水平状横向更新。而三倍体品种根茎形态并未表现出与二倍体品种明显的生长差异,可能原因及调控机制有待进一步研究。从根茎生长轮的组织结构特征来看,同一品种群内相同倍性的品种间根茎生长轮特征基本类似,而不同倍性的品种间差异较大;而品种群间不同品种染色体倍性与其根茎形态无明显关联,‘Coral Sunset’‘Prairie Charm’和‘Going Bananas’3个品种均为三倍体,但是根茎次生结构差异明显。因而,仅根据遗传倍性不能区分各品种的根茎生长轮特征。当然,由于生长轮的形成和发育受环境条件影响较大,加之芍药品种遗传背景复杂,关于生长轮的发育特性在芍药中的更普遍规律需要结合更多样本开展更进一步的研究。
根茎由于随着生长年限的增大,受限于材料的大小以及软硬程度的差异,采用组织切片的方法不能一一鉴别且花费时间较长,因而徒手切片结合体式显微观察可以作为多年生根茎年龄判别的快速方法。在生产实践中,我们可以通过上述徒手切片的一般操作方式快速地区分根茎与根,鉴定根茎的年龄结构。
4. 结 论
芍药不同品种地下根茎组织架构特征基本一致,且存在明显的龄级特征。二倍体及三倍体品种根茎形态发育特征相似,而与四倍体品种不同。不同芍药品种根茎次生结构均由周皮、皮层、次生韧皮部、形成层、次生木质部和中央髓组成,中国芍药及杂种芍药品种群品种根茎生长轮结构相似而与伊藤杂种差异明显,杂种芍药品种群内三倍体及四倍体品种根茎生长轮结构差异较大,根茎生长轮结构特征与其品种倍性无关。芍药根茎中存在生长轮,且其数目能够反映芍药的实际生长年限。
-
图 1 芍药根茎结构发育示意
1YR:1龄生根茎;2YR:2龄生根茎;3YR:3龄生根茎;4YR:4龄生根茎;5YR:5龄生根茎;RB:根茎芽;St:茎;AR:不定根。1YR, 1 year old rhizome; 2YR, 2 years old rhizome; 3YR, 3 years old rhizome; 4YR, 4 years old rhizome; 5YR, 5 years old rhizome; RB, rhizome bud; St, stem; AR, adventitious root.
Figure 1. Structural characteristic of rhizome of herbaceous peony
图 3 不同品种根茎解剖结构
a、b. ‘大富贵’根茎解剖结构;c、d. ‘粉玉奴’根茎解剖结构;e、f. ‘珊瑚落日’根茎解剖结构;g、h. ‘乳霜之愉’根茎解剖结构;i、j. ‘草原风情’根茎解剖结构;k、l. ‘抓狂的香蕉’根茎解剖结构;Pe:周皮;Sp:次生韧皮部;Vc:维管形成层;Sx:次生木质部;Pi:髓。标尺 = 1 000 μm。a, b, rhizome anatomy of ‘Dafugui’; c, d, rhizome anatomy of ‘Fenyunu’; e, f, rhizome anatomy of ‘Coral Sunset’; g, h, rhizome anatomy of ‘Cream Delight’; i, j, rhizome anatomy of ‘Prairie Charm’; k, l, rhizome anatomy of ‘Going Bananas’; Pe, periderm; Sp, secondary phloem; Vc, vascular cambium; Sx, secondary xylem; Pi, pith. Scale bar = 1 000 μm.
Figure 3. Anatomical structure of rhizomes of different cultivars of herbaceous peony
图 6 芍药根茎不同生长年限生长轮观察
a. 1龄生根茎;b. 2龄生根茎;c. 3龄生根茎;d. 4龄生根茎;e. 5龄生根茎;f、g. 6龄生根茎;h、i. 7龄生根茎;①. 第1个生长轮;②. 第2个生长轮;③. 第3个生长轮;④. 第4个生长轮;⑤. 第5个生长轮;⑥. 第6个生长轮;⑦. 第7个生长轮。标尺 = 1 000 μm。a, 1 year old rhizome; b, 2 years old rhizome; c, 3 years old rhizome; d, 4 years old rhizome; e, 5 years old rhizome; f, g, 6 years old rhizome; h, i, 7 years old rhizome; ①, the first growth ring; ②, the second growth ring; ③, the third growth ring; ④, the fourth growth ring; ⑤, the fifth growth ring; ⑥, the sixth growth ring; ⑦, the seventh growth ring. Scale bar = 1 000 μm.
Figure 6. Observation on the growth rings of the rhizome of herbaceous peony under a stereomicroscope
表 1 供试芍药品种信息
Table 1 Variety information of experimental materials
编号 No. 品种名称 Variety name 品种群分类 Classification of cultivar groups 倍性 Ploidy level 1 ‘种生粉’ ‘Zhongshengfen’ 中国芍药品种群 Lactiflora group 2n = 2x = 10 2 ‘粉玉奴’ ‘Fenyunu’ 中国芍药品种群 Lactiflora group 2n = 2x = 10 3 ‘珊瑚落日’ ‘Coral Sunset’ 杂种芍药品种群 Hybrid group 2n = 3x = 15 4 ‘乳霜之愉’ ‘Cream Delight’ 杂种芍药品种群 Hybrid group 2n = 4x = 20 5 ‘草原风情’ ‘Prairie Charm’ 伊藤杂种品种群 Itoh hybrid group 2n = 3x = 15 6 ‘抓狂的香蕉’ ‘Going Bananas’ 伊藤杂种品种群 Itoh hybrid group 2n = 3x = 15 -
[1] Schweingruber F, Poschlod P. Growth rings in herbs and shrubs: life span, age determination and stem anatomy[J]. Forest, Snow and Landscape Research, 2005, 79: 195−415.
[2] 张露丹, 李海燕, 杨允菲. 松嫩平原单优种群落斑块拂子茅种群构件的年龄结构[J]. 草业学报, 2016, 25(9):20−27. doi: 10.11686/cyxb2016065 Zhang L D, Li H Y, Yang Y F. Age structures of modules in a Calamagrostis epigejos population in single-species dominant patches in the Songnen Plain of China[J]. Acta Parataculturae Sinica, 2016, 25(9): 20−27. doi: 10.11686/cyxb2016065
[3] 焦德志, 黄曌月, 周婵, 等. 扎龙湿地异质生境芦苇种群根茎动态及年龄结构[J]. 生态学杂志, 2016, 35(4):888−895. Jiao D Z, Huang Z Y, Zhou C, et al. Rhizome dynamics and age structure of Phragmites australis population in heterogeneous habitats in Zhalong Wetland[J]. Chinese Journal of Ecology, 2016, 35(4): 888−895.
[4] 冯国华, 李雕, 杨静慧, 等. 不同盐碱地上彩叶树的年生长动态和生态反应[J]. 西南大学学报(自然科学版), 2019, 41(4):86−91. Feng G H, Li D, Yang J H, et al. Annual growth dynamics and ecological responses of color-leaf trees planted in different saline-alkali soils[J]. Journal of Southwest University(Natural Science Edition), 2019, 41(4): 86−91.
[5] 金晓明, 敖永贵, 艾琳, 等. 呼伦贝尔沙地米氏冰草种群构件年龄结构动态[J]. 草地学报, 2011, 19(3):367−371, 387. doi: 10.11733/j.issn.1007-0435.2011.03.001 Jin X M, Ao Y G, Ai L, et al. Age structures of Agropyron michnoi in Hulunbeier Sandy Grassland[J]. Acta Agrestia Sinica, 2011, 19(3): 367−371, 387. doi: 10.11733/j.issn.1007-0435.2011.03.001
[6] Montwé D, Isaac-Renton M, Hamann A, et al. Cold adaptation recorded in tree rings highlights risks associated with climate change and assisted migration[J/OL]. Nature communications, 2018, 9(1): 1574[2018−10−10]. https://www.nature.com/articles/s41467-018-04039-5.pdf.
[7] 杨允菲, 张宝田, 田尚衣. 松嫩平原旱地生境芦苇种群不同龄级根茎的干物质贮藏及水溶糖含量[J]. 应用生态学报, 2008, 19(9):1905−1910. Yang Y F, Zhang B T, Tian S Y. Dry matter storage and water soluble sugar content in different age classes rhizomes of Phragmites communis population in dry land habitat of Songnen Plain of China[J]. Chinese Journal of Applied Ecology, 2008, 19(9): 1905−1910.
[8] 周姣姣, 陶爱恩, 何正春, 等. 不同生长年限长柱重楼根茎中主要次生代谢产物的累积变化[J]. 黑龙江农业科学, 2018(3):121−125. Zhou J J, Tao A E, He Z C, et al. Changes of accumulation of main secondary metabolites in rhizome of Paris forrestii (Takht.) H.Li. of different growth years[J]. Heilongjiang Agricultural Sciences, 2018(3): 121−125.
[9] 赵飞亚, 陶爱恩, 董洪, 等. 不同生长年限南重楼主要次生代谢产物积累与其质量的关联性研究[J]. 时珍国医国药, 2018, 29(3):694−697. Zhao F Y, Tao A E, Dong H, et al. Research on the correlation between accumulation of main secondary metabolites and the quality of Paris vietnamensis with different growth years[J]. Lishizhen Medicine and Materia Medica Research, 2018, 29(3): 694−697.
[10] 徐苏男, 李虹仪, 李悦, 等. 养分异质条件下结缕草克隆分株生长、光合色素及叶绿素荧光特性的生理整合[J]. 江苏农业学报, 2018, 34(4):754−761. doi: 10.3969/j.issn.1000-4440.2018.04.006 Xu S N, Li H Y, Li Y, et al. Physiological integration of plant growth, photosynthetic pigment content and chlorophyll fluorescence characteristics of Zoysia japonica clonal ramets under nutrient heterogeneity[J]. Jiangsu Journal of Agricultural Sciences, 2018, 34(4): 754−761. doi: 10.3969/j.issn.1000-4440.2018.04.006
[11] 杨勇, 曾秀丽, 张姗姗, 等. 5种野生芍药在我国西南地区的地理分布与资源特点研究[J]. 四川农业大学学报, 2017, 35(1):69−74, 87. Yang Y, Zeng X L, Zhang S S, et al. Geographical distribution and resource characteristics of 5 wild peonies in Southwest China[J]. Journal of Sichuan Agricultural University, 2017, 35(1): 69−74, 87.
[12] Kamenetsky R, Dole J. Herbaceous peony (Paeonia): genetics, physiology and cut flower production[J]. Floriculture and Ornamental Biotechnology, 2012, 6: 62−77.
[13] 秦魁杰.芍药[M]. 北京: 中国林业出版社, 2014. Qin K J. Peony[M]. Beijing: China Forestry Publishing House, 2014.
[14] 李嘉珏. 中国牡丹与芍药[M]. 北京: 中国林业出版社, 1999. Li J J. Chinese tree peony and herbaceous peony[M]. Beijing: China Forestry Publishing House, 1999.
[15] 刘建鑫, 杨柳慧, 魏冬霞, 等. 芍药属组内组间杂交及部分后代核型分析与SSR鉴定[J]. 北京林业大学学报, 2017, 39(4):72−78. Liu J X, Yang L H, Wei D X, et al. Intrasectional and intersectional cross breeding of Paeonia and karyotype analysis and SSR identification of some hybrids[J]. Journal of Beijing Forestry University, 2017, 39(4): 72−78.
[16] 周学刚, 陈淑欣, 魏东华, 等. 不同种质和不同部位白芍原植物中芍药苷和芍药内酯苷的含量测定[J]. 医药导报, 2011, 30(11):1477−1480. doi: 10.3870/yydb.2011.11.029 Zhou X G, Chen S X, Wei D H, et al. Determination of paeoniflorin and albiflorin in different germplasms and parts of peony root[J]. Herald of Medicine, 2011, 30(11): 1477−1480. doi: 10.3870/yydb.2011.11.029
[17] 储姗姗, 查良平, 段海燕, 等. 中国芍药组7种植物根的生长轮及其在赤芍类药材鉴别中的应用[J]. 中国中药杂志, 2017, 42(19):3723−3727. Chu S S, Zha L P, Duan H Y, et al. Growth rings in roots of seven Sect. Paeonia species and its application on identification of Paeoniae Radix Rubra[J]. China Journal of Chinese Materia Medica, 2017, 42(19): 3723−3727.
[18] 张建军, 杨勇, 于晓南. 芍药根茎芽发育及更新规律的形态学研究[J]. 西北农业报, 2018, 27(7):1008−1016. Zhang J J, Yang Y, Yu X N. Morphological observation on the development and regeneration of rhizome buds in Paeonia lactiflora[J]. Acta Agriculturae Boreali-Occidentalis Sinica, 2018, 27(7): 1008−1016.
[19] 焦德志, 姜秋旭, 曹瑞, 等. 扎龙湿地不同生境芦苇种群根茎数量特征及动态[J]. 生态学报, 2018, 38(10):3432−3440. Jiao D Z, Jiang Q X, Cao R, et al. Quantitative characteristics and dynamics of the rhizome of Phragmites australis populations in the Zhalong Wetland[J]. Acta Ecologica Sinica, 2018, 38(10): 3432−3440.
[20] 黄云鹏, 王邦富, 范繁荣, 等. 林分类型及郁闭度对多花黄精根茎多糖含量的影响[J]. 中国农学通报, 2016, 32(10):102−105. doi: 10.11924/j.issn.1000-6850.casb15090114 Huang Y P, Wang B F, Fan F R, et al. Effect of forest types and canopy density on polysaccharide content of Polygonatum cyrtonem[J]. Chinese Agricultural Science Bulletin, 2016, 32(10): 102−105. doi: 10.11924/j.issn.1000-6850.casb15090114
[21] De Witte L C, Stöcklin J. Longevity of clonal plants: why it matters and how to measure it[J]. Annals of Botany, 2010, 106(6): 859−870. doi: 10.1093/aob/mcq191
[22] 许世磊. 芍药植株促芽的研究[D]. 泰安: 山东农业大学, 2012. Xu S L. The sdudy of promoting crown bud formation in herbaceous cultivars[D]. Tai ’an: Shandong Agricultural University, 2012.
[23] 黄永高, 金飚, 贾妮, 等. 芍药和牡丹部分品种茎叶器官的解剖学观察比较[J]. 江苏农业报, 2006, 22(4):447−451. Huang Y G, Jin B, Jia N, et al. Anatomical observation on Paeonia suffruticosa Andr. and Paeonia lactiflora Pall based on phytomorphology of stem and leaf[J]. Jiangsu Journal of Agricultural Sciences, 2006, 22(4): 447−451.
[24] 吕晔, 薛治慧, 吴改娥, 等. 三倍体和四倍体枣减数分裂行为异常现象观察[J]. 园艺学报, 2018, 45(4):659−668. Lü Y, Xue Z H, Wu G E, et al. Abnormal meiosis behaviors of triploid and tetraploid Chinese jujube[J]. Acta Horticulturae Sinica, 2018, 45(4): 659−668.
[25] 陈敏敏, 周音, 孙亿敬, 等. 秋水仙素诱导百合多倍体及流式细胞仪倍性鉴定研究[J]. 上海农业学报, 2018, 34(2):81−87. Chen M M, Zhou Y, Sun Y J, et al. Polyploidy induction of Lilium spp. by colchicine and ploidy identification by flow cytometry[J]. Acta Agriculturae Shanghai, 2018, 34(2): 81−87.
[26] 张磊. 高温诱导杜仲花粉染色体加倍技术研究[D]. 北京: 北京林业大学, 2010. Zhang L. Studies on pollen chromosome doubling technique of Eucommia ulmoides Oliv. by high temperature induction[D]. Beijing: Beijing Forestry University, 2010.
[27] 王沛琦. 白杨杂种三倍体离体快繁与六倍体诱导[D]. 北京: 北京林业大学, 2014. Wang P Q. Studies on rapid propagation and hexaploid induction of triploid hybrids of white poplar in vitro[D]. Beijing: Beijing Forestry University, 2014.
[28] Yang L L, Zhang J J, Da Silva J A T, et al. Variation in ploidy and karyological diversity in different herbaceous peony cultivar groups[J]. Journal of the American Society for Horticultural Science, 2017, 142(4): 272−278. doi: 10.21273/JASHS04015-17
[29] 马慧, 于晓南. 4个观赏芍药新品种染色体核型分析[J]. 河北农业大学学报, 2013, 36(6):61−65. Ma H, Yu X N. Studies on karyotypes of four cultivars in herbaceous peony[J]. Journal of Agricultural University of Hebei, 2013, 36(6): 61−65.
-
期刊类型引用(4)
1. 王慧娟,王二强,符真珠,李艳敏,王晓晖,袁欣,高杰,王利民,张和臣. 芍药根茎形成发育过程中内源激素和碳水化合物的变化. 河南农业科学. 2024(03): 118-124 .  百度学术
百度学术
2. 魏瑶,王娟,张岗,彭亮,颜永刚,陈莹. 不同年限黄芩根结构及黄酮类物质变化特征研究. 中南药学. 2023(01): 116-122 .  百度学术
百度学术
3. 董志君,高健洲,于晓南. 烯效唑对盆栽芍药生理特性及显微结构的影响. 北京林业大学学报. 2022(07): 117-125 .  本站查看
本站查看
4. 李艳敏,蒋卉,符真珠,张晶,袁欣,王慧娟,高杰,董晓宇,王利民,张和臣. 芍药花药愈伤组织诱导及体细胞胚发生. 植物学报. 2021(04): 443-450 .  百度学术
百度学术
其他类型引用(3)



 下载:
下载: