Dyeing process and mechanism of eucalyptus veneer with Dalbergia bariensis heartwood pigment as dye
-
摘要:目的从巴里黄檀心材中提取色素制得天然染料对桉木单板进行染色,在充分利用巴里黄檀加工剩余物的同时提高桉木的附加值。方法利用超高效液相色谱串联四极杆−静电场轨道阱高分辨质谱仪联用技术(UPLC-Q-EXCTIVE-MS)对巴里黄檀心材内含物的主要成分进行分析。探究桉木单板最佳上染工艺,测定染色单板的水洗色牢度。通过傅里叶变换红外光谱(FTIR)分析巴里黄檀染料与木材的结合方式,并使用场发射扫描电子显微镜(FESEM)观察染料在桉木单板内的分布特征,从而探究巴里黄檀染料对桉木单板的染色机理。结果采用UPLC-Q-EXCTIVE-MS法从巴里黄檀心材色素中鉴定出9种黄酮类和酚类成分:锦葵色素、鼠李黄素、紫铆查尔酮、樱花素、茜草素、木犀草素、苏木素、乔松素和花旗松素。桉木单板的最佳上染工艺为:染色温度90 ℃,染色时间12 h,色素质量分数4%,NaCl质量分数2%。染色试验因素影响的主次顺序为:温度 > 色素质量分数 > 染色时间 > NaCl质量分数,水洗对染色单板色牢度影响的主次顺序为:温度 > 色素质量分数 > 染色时间 > NaCl质量分数。通过成分分析结合FTIR与FESEM对染色机理进行分析,初步判定巴里黄檀染料结合桉木单板的机理为物理吸附和分子间氢键结合。结论利用巴里黄檀心材色素制成天然染料对桉木单板进行仿珍染色,为珍贵红木的充分利用开辟了新的途径,并为速生材高附加值利用作出了探索。Abstract:ObjectiveIn order to make full use of the processing residues of Dalbergia bariensis and improve the added value of eucalyptu, natural dye has been extracted from the Dalbergia bariensis heartwood.MethodThe main components of Dalbergia bariensis heartwood content were analyzed by ultra-high performance liquid chromatography with quadrupole-electrostatic field orbitrap high resolution mass spectrometry. The best dyeing process and color fastness were measured. The binding mechanism of Dalbergia bariensis dye and eucalyptus veneer was analyzed by Fourier transform infrared spectroscopy (FTIR).The distribution characteristics of the dye in eucalyptus veneer were observed by the field emission scanning electron microscope (FESEM).ResultNine phenols were identified from the pigment of Dalbergia bariensis heartwood by UPLC-Q-EXACTIVE-MS: malvidin, rhamnetin, butein, sakuranetin, alizarin, luteolin, hemotoxylin, pinocembrin, taxifolin. The optimal dyeing process of eucalyptus veneer was: dyeing temperature 90 ℃, dyeing time 12 hours, pigment mass fraction 4%, NaCl mass fraction 2%. The order of influencing factors of dyeing test was: temperature > pigment mass fraction > dyeing time > NaCl mass fraction. The order of influencing factors of color fastness was: temperature > pigment mass fraction > dyeing time > NaCl mass fraction. It was preliminarily determined as physical adsorption and intermolecular hydrogen bonding through the analysis of FTIR’s and FESEM’s reaction to the dyeing mechanism.ConclusionNatural dye extracted from Dalbergia bariensis heartwood is used to imitate the precious rosewood by dyeing eucalyptus veneer, which blazes a trail in the full utilization of precious rosewood and explores the high value-added utilization of fast-growing wood.
-
Keywords:
- Dalbergia bariensis /
- wood dyeing /
- natural dye /
- precious rosewood imitation /
- eucalyptus
-
巴里黄檀(Dalbergia bariensis),蝶形花科,黄檀属。因其优良的机械性能、瑰丽的色泽花纹而被广泛用于高档家具及工艺品的制作中[1],但因其成材缓慢、原产国限制出口等原因,巴里黄檀资源已逐步趋向枯竭,巴里黄檀加工过程中产生的刨花锯屑等废料常用作热源燃烧,造成了极大浪费,因此如何高效利用巴里黄檀小废料成为了待解决的问题。巴里黄檀心材乙醇抽提物呈红色,主要成分为黄酮类物质,国内外对于木材抽提物的利用多见于防腐[2],而染色方面研究较少,无法充分利用巴里黄檀心材抽提物作为色素应用的优势。
桉木(Eucalyptus robusta)是我国南方重要的速生用材树种,具有培育周期短、适应力强、纤维形态好等特点[3]。但也因其生长周期过短存在着颜色不均匀、色泽单一、纹理不清晰等缺陷,导致其附加值较低。木材染色是解决桉木颜色不均、色泽单一的较为直接的一种手段,国内外学者对此提出了不同的理论和方法。王春灿等[4]探究了酸性大红3R(Acid scarlet 3R)染料对杉木人工林木材的染色机理,但未考虑到酸性大红3R是偶氮染料,为含有苯环的高共轭分子,对环境水体的影响较大。李志勇等[5]探究了活性染料对樟子松(Pinus sylvestris)单板的染色工艺,但未探究单板染色后的色牢度。Tchinda等[6]从非洲紫檀(Pterocarpus soyauxii)中提取天然染料对木纤维织物进行染色,但未探究染料与织物的结合机理。当前研究中,木材染色所使用的染料多为人工合成染料,其加工过程中污染较大,且对人体有致癌风险[7-8]。天然染料具有生态友好,有益健康等优势,但关于天然染料对木材染色机理的探究较少。本研究主要对巴里黄檀心材加工剩余物进行提取和精制,获得天然染料,对桉木单板进行仿珍染色,使其具有红木色泽。在充分利用巴里黄檀资源的同时提高桉木附加值,并为同类红木的高效利用开辟了一条新途径。
1. 材料与方法
1.1 材 料
桉木单板(长30 mm × 宽30 mm × 厚1.7 mm)、巴里黄檀心材刨花(购于广西凭祥,经广西大学林学院符韵林教授鉴定为蝶形花科黄檀属巴里黄檀的心材部分)、壳聚糖(脱乙酰度80.0% ~ 95.0%)、冰醋酸、氯化钠、无水乙醇、无水硫酸钠。以上试剂均为国产分析纯。
1.2 研究方法
1.2.1 壳聚糖溶液的配制
依照Yasuhiko[9]的壳聚糖溶液配制方法,将2 g壳聚糖加入到100 mL蒸馏水中,室温静置1 h后,加入冰醋酸0.5 g,60 ℃恒温搅拌至壳聚糖充分溶解,形成稳定均一溶液。置于超声波清洗机中消泡20 min。
1.2.2 桉木单板预处理
将配好的壳聚糖溶液用刷子涂刷在气干桉木单板表面,于室温下通风干燥。
1.2.3 巴里黄檀染料制备
将巴里黄檀心材刨花粉碎,过80目筛,以70%乙醇,料液质量比1∶20,60 ℃恒温搅拌提取4 ~ 7 h,过滤得浸提液,减压浓缩除去乙醇,烘干水分,得到巴里黄檀心材色素浸膏,以一定比例溶解于50%乙醇溶液中,按一定比例加入助染剂NaCl,充分溶解后得到巴里黄檀染料。密封避光保存。
1.2.4 桉木单板上染正交试验
以染色时间、温度、NaCl质量分数、色素质量分数作为考察因素,采用L16(45)正交表,染色因素水平见表1。每组试件10片,染料150 mL,重复3次。色差计为ADCI系列全自动色差仪。测量木材染色前后表面的色差值,工作原理是使用光学系统测试样品的三刺激值,通过对数值进行积分,得到颜色的数学表达式。通过对试样变化前后三刺激值进行计算,得出不同试样的染色前后色差,即被测样品的色差值[10]。色差值越大,染色越深,染色效果越好。本实验以色差值为响应值评定桉木上染效果。
表 1 正交试验因素和水平Table 1. Factor and level of orthogonal experiment水平
Level温度
Temperature/℃染色时间
Dyeing time/h色素质量分数
Pigment mass fraction/%NaCl 质量分数
NaCl mass fraction/%1 60 8 1.0 1.0 2 70 10 2.0 1.5 3 80 12 3.0 2.0 4 90 14 4.0 2.5 1.3 染色单板水洗色牢度测定
以染色单板水洗前后的色差值大小作为其耐水洗色牢度强弱的考察指标[11]。将已测量过颜色的每组染色单板分别放入装有100 mL蒸馏水的烧杯中,60 ℃恒温水浴加热2 h,取出木片于室温下风干,再测量其表面颜色,计算水洗处理前后色差。
1.4 染色机理分析
1.4.1 巴里黄檀心材色素化学成分分析
采用超高效液相色谱串联四极杆−静电场轨道阱高分辨质谱仪联用技术(UPLC-Q-EXCTIVE-MS)对巴里黄檀心材色素成分进行分离鉴定。超高效液相色谱(UPLC)条件:色谱柱为ACQUITY UPLCBEHC18色谱柱(50 mm × 2.1 mm,1.7 μm),柱温30 ℃;正离子(ESI+)模式;流动相A为0.1%甲酸水,流动相B为甲醇。自动进样器温度4 ℃,进样体积1 μL。样品梯度洗脱程序见表2。
表 2 样品流动相梯度洗脱条件Table 2. Gradient elution conditions of mobile phase for samples洗脱时间
Elution
time/min流速
Flow velocity/
(mL·min− 1)流动相 A
Mobile
phase A流动相 B
Mobile
phase B0 0.3 95%CH2OH 5%CH3OH 2.0 0.3 95%CH2OH 5%CH3OH 20.0 0.3 5%CH2OH 95%CH3OH 22.0 0.3 5%CH2OH 95%CH3OH 22.1 0.3 95%CH2OH 5%CH3OH 26.0 0.3 95%CH2OH 5%CH3OH 高分辨质谱(Q-EXACTIVE-MS)条件:加热型电喷雾(HESI)离子源;温度300 ℃;传输毛细管温度320 ℃;鞘气206 000 Pa;辅助气流速69 kPa;正离子和负离子模式下喷雾电压均为3.0 kV;扫描模式为Full MS和Full MS/dd-MS2,质量范围100 ~ 500 amu,一级扫描和二级扫描分辨率分别为70 000、17 500。
1.4.2 桉木单板染色前后傅里叶变换红外光谱分析
FTIR仪器为德国BRUKER公司TENSOR Ⅱ型傅里叶变换红外光谱仪。刮取未处理单板、壳聚糖预处理单板、染色单板少许,与KBr一起放入60 ℃烘箱中干燥24 h,冷却后将试样与KBr以质量比1∶100比例混合,研磨均匀,压片,进行红外光谱测试。光谱仪的分辨率为1 cm− 1,扫描波数范围为400 ~ 4 000 cm− 1,扫描次数32次。
1.4.3 场发射扫描电子显微镜观察
将未处理单板和染色单板烘干,取横切面与弦切面试样,使用切片机(德国Leica公司SM2400型平推式切片机)刨光表面,用双面导电胶将试样贴在样品台上,喷金处理后,使用日本日立S-3400N型场发射扫描电子显微镜观察样品微观形貌。
2. 结果与分析
2.1 巴里黄檀心材色素成分分析
采用超高效液相色谱串联四极杆–静电场轨道阱高分辨质谱仪联用技术(UPLC-Q-EXCTIVE-MS)对巴里黄檀心材色素成分进行分析,从中分离鉴定出9种黄酮类与酚类化合物,分别为锦葵色素、鼠李黄素、紫铆查尔酮、樱花素、茜草素、木犀草素、苏木素、乔松素和花旗松素。根据各成分的保留时间与质谱信息,通过与mzcloud比对二级质谱信息(见表3),结合文献的比对,推测出各化合物可能的裂解途径。
表 3 巴里黄檀心材色素中化学成分离子结构信息Table 3. Characterization of chemical constituents in pigment from Dalbergia bariensis heartwood by UPLC-Q-exactive orbitrap-MS峰号
No.保留时间
Retention time/min分子式
Formula实测分子量
Experimental
molecalar mass理论分子量
Theoretical
molecalar mass二级离子分子量
Product ion molecular mass化学成分
Chemical component文献来源
Reference1 5.493 C17H14O7 331.379 [M+H]+331.072 316.056, 299.053, 271.058 锦葵色素
MalvidinGómez-Ariza, et al[12] 2 5.790 C16H12O7 317.064 [M+H]+317.057 302.041, 285.038, 257.020 鼠李黄素
RhamnetinSilvio, et al[13] 3 6.094 C15H12O5 273.074 [M+H]+273.067 255.063, 227.069, 199.074 紫铆查尔酮
ButeinCheng, et al[14] 4 6.688 C16H14O5 287.090 [M+H]+287.082 167.033, 121.064 樱花素
SakuranetinYan, et al[15] 5 7.494 C14H8O4 241.048 [M+H]+241.041 131.048 茜草素
AlizarinHan, et al[16] 6 7.561 C15H10O6 285.088 [M-H]− 285.046 257.044, 213.064 木犀草素
Luteolin于小杰等[17]
Yu X J, et al.[17]7 7.789 C16H14O6 301.069 [M-H]− 301.077 124.014, 177.016 苏木素
Hemotoxylin李争春等[18]
Li Z C, et al[18]8 10.214 C15H12O4 257.079 [M+H]+257.072 229.084, 211.075 乔松素
Pinocembrin王伟楠等[19]
Wang W N, et al.[19]9 10.915 C15H12O7 305.064 [M+H]+305.057 153.054 花旗松素
TaxifolinMa, et al[20] 注:本试验中使用的两种模式都是1个电荷,这时的质荷比(m/z)就等于分子量。Notes: the two modes used in this experiment are one charge, and the mass to charge ratio (m/z) is equal to the molecular mass. 化合物1的裂解途径为:该化合物准分子离子[M+H]+(m/z 331.379)脱去1个甲基得到碎片离子C16H12O7(m/z 316.056),进一步脱去1个羟基得碎片离子C16H11O6(m/z 299.053),再脱去1分子CO得碎片离子C15H11O5(m/z 271.058),结合文献报道[12],化合物1被鉴定为锦葵色素。
化合物2的裂解途径为:该化合物准分子离子[M+H]+(m/z 317.064)脱去1个甲基得到碎片离子C15H10O7(m/z 302.041),进一步脱去1个羟基得碎片离子C15H9O6(m/z 285.038),再脱去1分子CO得碎片离子C14H9O5(m/z 257.020),结合文献报道[13],化合物2被鉴定为鼠李黄素。
化合物3的裂解途径为:该化合物准分子离子[M+H]+(m/z 273.074)脱去1个水分子得到碎片离子C15H11O4(m/z 255.063),进一步脱去1分子CO得碎片离子C14H11O3(m/z 227.069),再脱去1分子CO得碎片离子C13H11O2(m/z 199.074),结合文献报道[14], 化合物3被鉴定为紫铆查尔酮。
化合物4的裂解途径为:该化合物准分子离子[M+H]+(m/z 287.090)直接经过RetroDiels-Alder (RDA)裂解获得碎片离子C8H7O4(m/z 167.033)和C8H9O1(m/z 121.064),结合文献报道[15],化合物4被鉴定为樱花素。
化合物5的裂解途径为:该化合物准分子离子[M+H]+(m/z 241.048)直接经过RDA裂解获得碎片离子C8H3O2(m/z 131.048),结合文献报道[16], 化合物5被鉴定为茜草素。
化合物6的裂解途径为:该化合物准分子离子[M-H]−(m/z 285.088)脱去1分子CO得到碎片离子C14H9O5(m/z 257.044),又进一步脱去1分子CO2得碎片离子C13H9O3(m/z 213.064),结合文献报道[17],化合物6被鉴定为木犀草素。
化合物7的裂解途径为:该化合物准分子离子[M-H]−(m/z 301.069)裂解为碎片离子C6H4O3(m/z 124.014)和碎片离子C10H9O3(m/z 177.016),结合文献报道[18],化合物7被鉴定为苏木素。
化合物8的裂解途径为:该化合物准分子离子[M+H]+(m/z 257.079)脱去1分子CO得到碎片离子C14H13O3(m/z 229.084),进一步脱去1个水分子得到碎片离子C14H11O2(m/z 211.075),结合文献报道[19],化合物8被鉴定为乔松素。
化合物9的裂解途径为:该化合物准分子离子[M+H]+(m/z 305.064)直接经过RDA裂解获得碎片离子C8H9O3(m/z 153.054),结合文献报道[20],化合物9被鉴定为花旗松素。
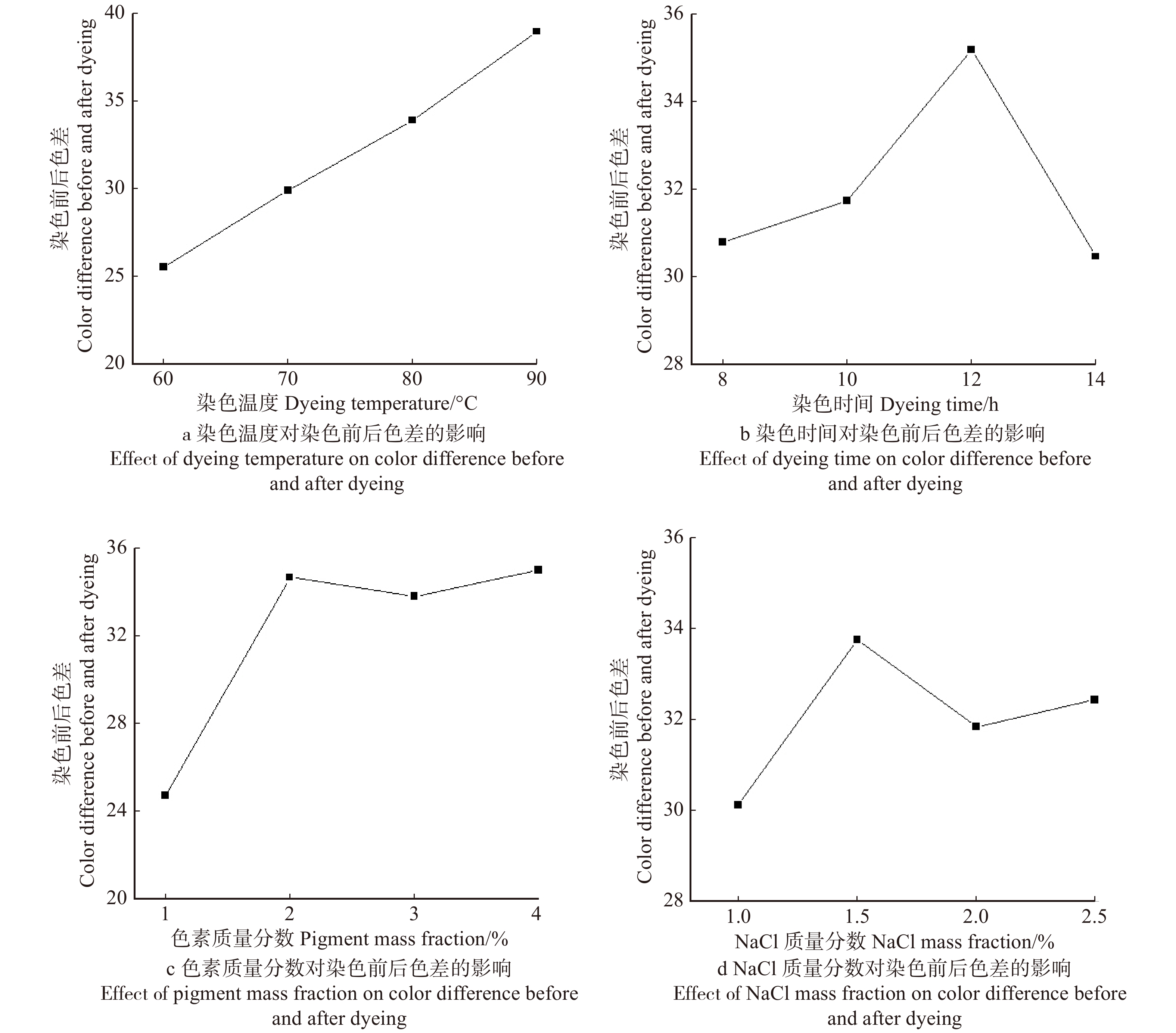
2.2 主要因素对染色效果的影响
根据正交试验设计表对桉木进行染色处理试验,测量染色前后色差,试验结果见表4。对染色结果进行数据分析,各因子影响染色的主次顺序为:温度 > 色素质量分数 > 染色时间 > NaCl质量分数。最佳染色条件为:染色温度为90 ℃,染色时间为12 h,色素质量分数为4%,NaCl质量分数为2%。图1为试验样品染色效果图和巴里黄檀实物图的对比,经图1观察对比,15号样品与巴里黄檀颜色最为接近。
表 4 染色前后色差对比结果Table 4. Comparison results of color differences before and after dyeing序号
No.染色温度
Dyeing temperature/℃染色时间
Dyeing time/h色素质量分数
Pigment mass fraction/%NaCl质量分数
NaCl mass fraction/%空列
Null column染色前后色差
Color difference before
and after dyeing1 60 8 1 1.0 1 17.43 2 60 10 2 1.5 2 30.77 3 60 12 3 2.0 3 27.12 4 60 14 4 2.5 4 26.38 5 70 8 2 2.0 4 30.23 6 70 10 1 2.5 3 19.64 7 70 12 4 1.0 2 35.40 8 70 14 3 1.5 1 34.26 9 80 8 3 2.5 2 36.12 10 80 10 4 2.0 1 38.78 11 80 12 1 1.5 4 30.60 12 80 14 2 1.0 3 30.02 13 90 8 4 1.5 3 39.35 14 90 10 3 1.0 4 37.65 15 90 12 2 2.5 1 47.57 16 90 14 1 2.0 2 31.20 均值1
Average 1 (k1)25.524 30.782 24.717 30.125 34.497 均值2
Average 2 (k2)29.882 31.732 34.647 33.745 33.372 均值3
Average 3 (k3)33.880 35.172 33.787 31.832 29.032 均值4
Average 4 (k4)38.942 30.465 34.977 32.427 31.215 极差
Range (R)13.418 4.707 10.260 3.620 5.465 2.3 工艺参数对桉木单板染色效果的影响
2.3.1 温度对染色效果的影响
如图2a所示,温度对桉木单板上染效果影响较大。随着温度的升高,试件染色前后色差逐渐升高,色差值由25.524上升到38.942,根据扩散原理,温度越高,扩散系数越大,分子扩散的速度越快[21]。因而染料中黄酮和酚类化合物在木材导管和纹孔中扩散的速度加快,试件颜色变深。当温度升高到90 ℃时,木材的色差值达到最高,此时染色最深。染色最佳温度为90 ℃。
2.3.2 染色时间对染色效果的影响
如图2b所示,染色时间8 ~ 10 h时试件染色前后色差值基本不变,10 h以后色差值逐渐升高。随着染色时间的延长,染料中的色素逐渐渗透到试件的内部结构中,但固着仍需较长时间。当染料与木纤维结合达到饱和后,继续煮染可能破坏已形成的分子间作用力。故12 h桉木的色差值最大,最佳的染色时间为12 h。
2.3.3 色素质量分数对染色效果的影响
如图2c所示,试件染色前后色差值随色素质量分数的升高而升高,即试件颜色随染液质量分数上升而加深。随着色素质量分数的升高,色素与木纤维接触面积增大,有助于木材对染液的吸收进而提高染色效果。色素的最佳质量分数为4%。
2.3.4 NaCl质量分数对染色效果的影响
如图2d所示,随着NaCl质量分数的增加,试件染色前后色差增大,即试件颜色随NaCl质量分数上升而加深。在木材染色的过程中,NaCl可以提供显正电性钠离子[22],它可减小木纤维表面与染料中黄酮和酚类化合物之间的负电荷排斥力,有效减少木材表面对染料阴离子的阻染作用,使得染料分子进一步接近木纤维表面,从而提高染色上染率。NaCl最佳质量分数为2%。
2.4 色牢度检测
桉木染色单板水洗前后色差结果见表5。色差值变化相对较小,说明色牢度较好[11]。本试验中染色材水洗前后平均色差为5.55,色差变化较小,故染色材色牢度相对较好。各因素对试验样品色牢度的影响的顺序为:温度 > 色素质量分数 > 染色时间 > NaCl质量分数。巴里黄檀染料中黄酮和酚类化合物与木纤维之间主要以分子间氢键和吸附作用结合,染色过程中,高温能提供较高的活化能,加剧分子间运动,并破坏分子间结合力[23]。其他3个因素对试件水洗色牢度的影响均小于温度。因此桉木单板染色牢度的主要影响因素为温度,桉木单板在温度70 ℃、染色时间10 h、色素质量分数4%、NaCl质量分数2.5%时,耐水洗色牢度最大。
表 5 染色样品水洗前后色差对比结果Table 5. Comparison results of color differences before and after washing序号
No.染色温度
Dyeing temperature/℃染色时间
Dyeing time/h色素质量分数
Pigment mass fraction/%NaCl质量分数
NaCl mass fraction/%空列
Null column水洗前后色差
Color difference before
and after washing1 60 8 1 1.0 1 7.99 2 60 10 2 1.5 2 6.90 3 60 12 3 2.0 3 6.63 4 60 14 4 2.5 4 5.37 5 70 8 2 2.0 4 4.31 6 70 10 1 2.5 3 2.92 7 70 12 4 1.0 2 5.56 8 70 14 3 1.5 1 2.40 9 80 8 3 2.5 2 5.36 10 80 10 4 2.0 1 6.62 11 80 12 1 1.5 4 7.44 12 80 14 2 1.0 3 6.42 13 90 8 4 1.5 3 6.77 14 90 10 3 1.0 4 2.67 15 90 12 2 2.5 1 5.99 16 90 14 1 2.0 2 5.31 均值1
Average 1 (k1)6.733 6.133 5.915 5.660 5.750 均值2
Average 2 (k2)3.798 4.788 5.905 5.888 5.818 均值3
Average 3 (k3)6.485 6.405 4.290 5.718 5.685 均值4
Average 4 (k4)5.185 4.875 6.080 4.935 4.948 极差
Range (R)2.935 1.617 1.790 0.953 0.870 2.5 桉木染色处理前后FTIR分析
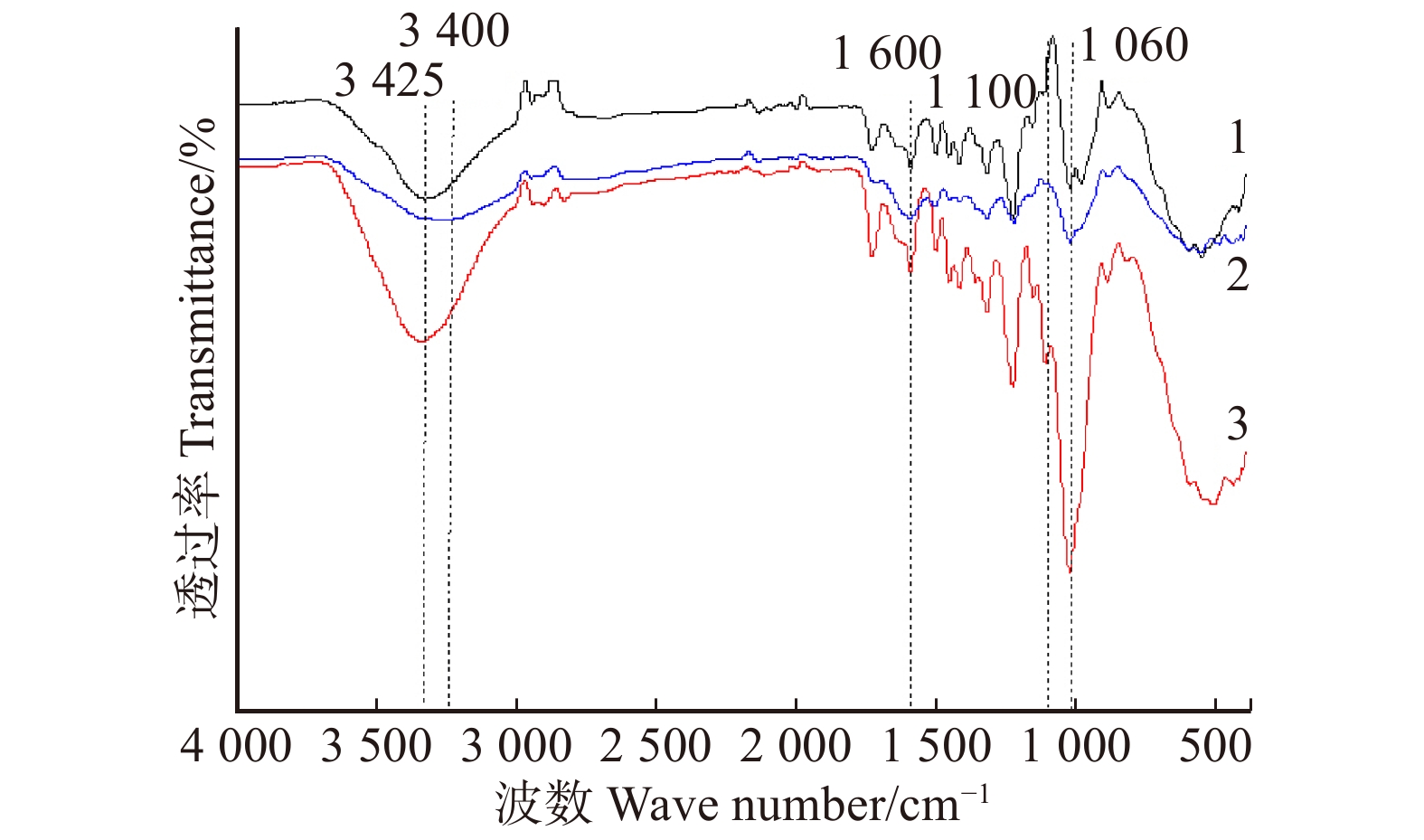
如图3所示,染色材在3 425 cm− 1处为木材纤维素中O–H的伸缩振动峰,染色后纤维素中O–H的伸缩振动降低,说明巴里黄檀染料和壳聚糖覆盖桉木纤维素中的O–H基团以及O–H的伸缩振动吸收峰与壳聚糖中N–H的伸缩振动吸收峰重叠而成的多重吸收峰的双重作用[24]。另外O–H特征频率向低波数移动,说明纤维素和半纤维素与黄酮类化合物之间形成分子间氢键使木材上吸附了大量巴里黄檀染料,使得 O–H(1 060 cm− 1)吸收峰增加,说明巴里黄檀染料与桉木表面结合良好,壳聚糖和NaCl可以减少桉木单板表面带有的负电荷,从而减少染色过程中负电荷对染料阴离子的排斥力,提高上染率。
2.6 桉木染色处理前后场发射扫描电子显微镜分析
未处理桉木木材横切面中可观察到排列整齐的圆形管孔(图4a),放大后观察到圆形管孔和相连生的轴向薄壁细胞(图4b)。弦切面观察到成排的轴向导管(图4d),放大后看到弧形导管壁和纹孔结构(图4e)。经过壳聚糖和NaCl处理后,可有效减少木材表面带有的负电荷,使得染料和木材能稳定结合。经过染色处理后,观察到木材横切面中染料部分覆盖导管,其旁边的轴向薄壁组织被完全覆盖,表面有较好的平整度(图4c)。在染色材的弦切面中,染料附着于轴向导管上(图4f)。说明染料中黄酮和酚类化合物与木质纤维形成分子间氢键,稳定结合在木材表面。
3. 结 论
(1)采取UPLC-Q-EXACTIVE-MS法从巴里黄檀心材色素中分离鉴定出9种黄酮类与酚类化合物,分别为锦葵色素、鼠李黄素、紫铆查尔酮、樱花素、茜草素、木犀草素、苏木素、乔松素和花旗松素。
(2) 使用壳聚糖溶液对桉木表面进行涂刷预处理,从巴里黄檀心材中提取色素制成天然染料,对桉木单板进行染色处理。各因子影响染色效果的主次顺序为:温度 > 色素质量分数 > 染色时间 > NaCl质量分数。通过正交试验分析得出最佳染色工艺参数为:温度90 ℃,色素质量分数4%,NaCl质量分数2%,染色时间12 h。对染色试件进行水洗色牢度测定,各因素对样品色牢度的影响顺序为:温度 > 色素质量分数 > 染色时间 > NaCl质量分数。影响染色样品色牢度的主要因素是温度,耐水洗最佳染色工艺为:温度70 ℃,染色时间10 h,色素质量分数4%,NaCl质量分数2.5%。
(3) 通过巴里黄檀心材色素成分检测结合红外与电镜分析得到染色机理为:巴里黄檀染料通过物理吸附和分子间氢键作用稳定结合在木材横切面的轴向薄壁组织和弦切面的导管中。
-
表 1 正交试验因素和水平
Table 1 Factor and level of orthogonal experiment
水平
Level温度
Temperature/℃染色时间
Dyeing time/h色素质量分数
Pigment mass fraction/%NaCl 质量分数
NaCl mass fraction/%1 60 8 1.0 1.0 2 70 10 2.0 1.5 3 80 12 3.0 2.0 4 90 14 4.0 2.5 表 2 样品流动相梯度洗脱条件
Table 2 Gradient elution conditions of mobile phase for samples
洗脱时间
Elution
time/min流速
Flow velocity/
(mL·min− 1)流动相 A
Mobile
phase A流动相 B
Mobile
phase B0 0.3 95%CH2OH 5%CH3OH 2.0 0.3 95%CH2OH 5%CH3OH 20.0 0.3 5%CH2OH 95%CH3OH 22.0 0.3 5%CH2OH 95%CH3OH 22.1 0.3 95%CH2OH 5%CH3OH 26.0 0.3 95%CH2OH 5%CH3OH 表 3 巴里黄檀心材色素中化学成分离子结构信息
Table 3 Characterization of chemical constituents in pigment from Dalbergia bariensis heartwood by UPLC-Q-exactive orbitrap-MS
峰号
No.保留时间
Retention time/min分子式
Formula实测分子量
Experimental
molecalar mass理论分子量
Theoretical
molecalar mass二级离子分子量
Product ion molecular mass化学成分
Chemical component文献来源
Reference1 5.493 C17H14O7 331.379 [M+H]+331.072 316.056, 299.053, 271.058 锦葵色素
MalvidinGómez-Ariza, et al[12] 2 5.790 C16H12O7 317.064 [M+H]+317.057 302.041, 285.038, 257.020 鼠李黄素
RhamnetinSilvio, et al[13] 3 6.094 C15H12O5 273.074 [M+H]+273.067 255.063, 227.069, 199.074 紫铆查尔酮
ButeinCheng, et al[14] 4 6.688 C16H14O5 287.090 [M+H]+287.082 167.033, 121.064 樱花素
SakuranetinYan, et al[15] 5 7.494 C14H8O4 241.048 [M+H]+241.041 131.048 茜草素
AlizarinHan, et al[16] 6 7.561 C15H10O6 285.088 [M-H]− 285.046 257.044, 213.064 木犀草素
Luteolin于小杰等[17]
Yu X J, et al.[17]7 7.789 C16H14O6 301.069 [M-H]− 301.077 124.014, 177.016 苏木素
Hemotoxylin李争春等[18]
Li Z C, et al[18]8 10.214 C15H12O4 257.079 [M+H]+257.072 229.084, 211.075 乔松素
Pinocembrin王伟楠等[19]
Wang W N, et al.[19]9 10.915 C15H12O7 305.064 [M+H]+305.057 153.054 花旗松素
TaxifolinMa, et al[20] 注:本试验中使用的两种模式都是1个电荷,这时的质荷比(m/z)就等于分子量。Notes: the two modes used in this experiment are one charge, and the mass to charge ratio (m/z) is equal to the molecular mass. 表 4 染色前后色差对比结果
Table 4 Comparison results of color differences before and after dyeing
序号
No.染色温度
Dyeing temperature/℃染色时间
Dyeing time/h色素质量分数
Pigment mass fraction/%NaCl质量分数
NaCl mass fraction/%空列
Null column染色前后色差
Color difference before
and after dyeing1 60 8 1 1.0 1 17.43 2 60 10 2 1.5 2 30.77 3 60 12 3 2.0 3 27.12 4 60 14 4 2.5 4 26.38 5 70 8 2 2.0 4 30.23 6 70 10 1 2.5 3 19.64 7 70 12 4 1.0 2 35.40 8 70 14 3 1.5 1 34.26 9 80 8 3 2.5 2 36.12 10 80 10 4 2.0 1 38.78 11 80 12 1 1.5 4 30.60 12 80 14 2 1.0 3 30.02 13 90 8 4 1.5 3 39.35 14 90 10 3 1.0 4 37.65 15 90 12 2 2.5 1 47.57 16 90 14 1 2.0 2 31.20 均值1
Average 1 (k1)25.524 30.782 24.717 30.125 34.497 均值2
Average 2 (k2)29.882 31.732 34.647 33.745 33.372 均值3
Average 3 (k3)33.880 35.172 33.787 31.832 29.032 均值4
Average 4 (k4)38.942 30.465 34.977 32.427 31.215 极差
Range (R)13.418 4.707 10.260 3.620 5.465 表 5 染色样品水洗前后色差对比结果
Table 5 Comparison results of color differences before and after washing
序号
No.染色温度
Dyeing temperature/℃染色时间
Dyeing time/h色素质量分数
Pigment mass fraction/%NaCl质量分数
NaCl mass fraction/%空列
Null column水洗前后色差
Color difference before
and after washing1 60 8 1 1.0 1 7.99 2 60 10 2 1.5 2 6.90 3 60 12 3 2.0 3 6.63 4 60 14 4 2.5 4 5.37 5 70 8 2 2.0 4 4.31 6 70 10 1 2.5 3 2.92 7 70 12 4 1.0 2 5.56 8 70 14 3 1.5 1 2.40 9 80 8 3 2.5 2 5.36 10 80 10 4 2.0 1 6.62 11 80 12 1 1.5 4 7.44 12 80 14 2 1.0 3 6.42 13 90 8 4 1.5 3 6.77 14 90 10 3 1.0 4 2.67 15 90 12 2 2.5 1 5.99 16 90 14 1 2.0 2 5.31 均值1
Average 1 (k1)6.733 6.133 5.915 5.660 5.750 均值2
Average 2 (k2)3.798 4.788 5.905 5.888 5.818 均值3
Average 3 (k3)6.485 6.405 4.290 5.718 5.685 均值4
Average 4 (k4)5.185 4.875 6.080 4.935 4.948 极差
Range (R)2.935 1.617 1.790 0.953 0.870 -
[1] 霄迪. 巴里黄檀[J]. 家具与室内装饰, 2013(3):96−101. doi: 10.3969/j.issn.1006-8260.2013.03.023 Xiao D. Dalbergia bariensis[J]. Furniture and Interior Decoration, 2013(3): 96−101. doi: 10.3969/j.issn.1006-8260.2013.03.023
[2] Kirker G T, Blodgett A B, Arango R A, et al. The role of extractives in naturally durable wood species[J]. International Biodeterioration & Biodegradation, 2013, 82: 53−58.
[3] 黄海文. 桉树的生长特性与种植管理技术[J]. 绿色科技, 2019(5):105−106. Huang H W. Growth characteristics and planting management techniques of eucalyptus[J]. Green Science and Technology, 2019(5): 105−106.
[4] 王春灿, 邓邵平, 林金国. 杉木人工林木材酸性染料染色性能[J]. 森林与环境学报, 2018, 38(1):111−117. Wang C C, Deng S P, Lin J G. Dyeing properties of Cunninghamia lanceolata wood with acid dye[J]. Journal of Forest and Environment, 2018, 38(1): 111−117.
[5] 李志勇, 郭明辉, 刘宇, 等. 活性染料对樟子松单板染色性能的影响[J]. 东北林业大学学报, 2013, 41(6):120−123. doi: 10.3969/j.issn.1000-5382.2013.06.028 Li Z Y, Guo M H, Liu Y, et al. Influence of reactive dye on dyeing performance of veneer for Pinus sylvest var. mongolica[J]. Journal of Northeast Forestry University, 2013, 41(6): 120−123. doi: 10.3969/j.issn.1000-5382.2013.06.028
[6] Tchinda J B S, Pétrissans A, Molina S, et al. Study of the feasibility of a natural dye on cellulosic textile supports by red padouk (Pterocarpus soyauxii) and yellow movingui (Distemonanthus benthamianus) extracts[J]. Industrial Crops & Products, 2014, 60: 291−297.
[7] Parmar R S, Singh C. A comprehensive study of eco-friendly natural pigment and its applications[J]. Biochemistry and Biophysics Reports, 2018, 13: 22−26. doi: 10.1016/j.bbrep.2017.11.002
[8] Heather M. Pigment toxicity[J]. ICCM Bulletion, 1977, 3(2): 11−17. doi: 10.1179/iccm.1977.3.2.004
[9] Yasuhiko H. Modification of wood by treatment with chitosan (ii.): comparison of the colour of chitosan-coated and thereafter stained surface of wood with that of the directly stained surface of wood[J]. Painting Technology, 1988, 23(12): 470−477.
[10] Apetrei C, Apetrei I M, Villanueva S, et al. Combination of an e-nose, an e-tongue and an e-eye for the characterisation of olive oils with different degree of bitterness[J]. Analytica Chimica Acta, 2010, 663(1): 91−97. doi: 10.1016/j.aca.2010.01.034
[11] Dubas S T, Chutchawalkulchai E, Egkasit S, et al. Deposition of polyelectrolyte multilayers to improve the color fastness of silk[J]. Textile Research Journal, 2007, 77(6): 437−441. doi: 10.1177/0040517507071969
[12] Gómez-Ariza J L, García-Barrera T, Lorenzo F. Anthocyanins profile as fingerprint of wines using atmospheric pressure photoionisation coupled to quadrupole time-of-flight mass spectrometry[J]. Analytica Chimica Acta, 2006, 570(1): 101−108. doi: 10.1016/j.aca.2006.04.004
[13] Silvio K, Uroš G, Tanja C, et al. The determination of phenolic profiles of Serbian unifloral honeys using ultra-high-performance liquid chromatography/high resolution accurate mass spectrometry[J]. Food Chemistry, 2013, 138(1): 32−40. doi: 10.1016/j.foodchem.2012.10.025
[14] Cheng X L, Wan J Y, Li P, et al. Ultrasonic/microwave assisted extraction and diagnostic ion filtering strategy by liquid chromatography-quadrupole time-of-flight mass spectrometry for rapid characterization of flavonoids in Spatholobus suberectus[J]. Journal of Chromatography A, 2011, 1218(34): 5774−5786. doi: 10.1016/j.chroma.2011.06.091
[15] Yan M, Chen M, Zhou F, et al. Separation and analysis of flavonoid chemical constituents in flowers of Juglans regia L. by ultra-high-performance liquid chromatography-hybrid quadrupole time-of-flight mass spectrometry[J]. Journal of Pharmaceutical and Biomedical Analysis, 2019, 164: 734−741. doi: 10.1016/j.jpba.2018.11.029
[16] Han J, Wanrooij J, Van Bommel M, et al. Characterisation of chemical components for identifying historical Chinese textile dyes by ultra high performance liquid chromatography-photodiode array-electrospray ionisation mass spectrometer[J]. Journal of Chromatography A, 2017, 1479: 87−96. doi: 10.1016/j.chroma.2016.11.044
[17] 于小杰, 岳贵娟, 薛梦, 等. HPLC-ESI-LTQ-Orbitrap分析芪归银方中黄酮成分体内代谢过程[J]. 质谱学报, 2017, 38(1):116−126. Yu X J, Yue G J, Xue M, et al. Metabolic process of flavonoids in Qi-gui-yingranule (QGY) based on HPLC-ESI-LTQ-Orbitrap[J]. Journal of Chinese Mass Spectrometry Society, 2017, 38(1): 116−126.
[18] 李争春, 郭彩霞, 白宝清, 等. 苏木水提物化学组成分析及有效成分的纯化结构表征[J]. 中草药, 2014, 45(8):1063−1067. Li Z C, Guo C X, Bai B Q, et al. Component analysis, and purification and characterization of active ingredients in aqueous extract from Sappan lignum[J]. Chinese Traditional and Herbal Drugs, 2014, 45(8): 1063−1067.
[19] 王伟楠, 孙光伟, 隋殿军. 蜂胶总黄酮滴丸的高分辨液质联用分析研究[J]. 吉林中医药, 2015, 35(12):1272−1274. Wang W N, Sun G W, Sui D J. HPLC-HRMS analysis of propolis total flavonoids dripping pills[J]. Jilin Journal of Traditional Chinese Medicine, 2015, 35(12): 1272−1274.
[20] Ma H, Liu Y, Mai X, et al. Identification of the constituents and metabolites in rat plasma after oral administration of Huanglian Shangqing pills by ultra high-performance liquid chromatography/quadrupole time-of-flight mass spectrometry[J]. Journal of Pharmaceutical & Biomedical Analysis, 2016, 125: 194−204.
[21] 巫若子. 微波对棉织物活性染料染色的作用机理探讨[J]. 染整技术, 2016, 38(6):15−18. doi: 10.3969/j.issn.1005-9350.2016.06.004 Wu R Z. Mechanism of microwave on cotton fabric with reactive dye[J]. Dyeing & Finishing Technology, 2016, 38(6): 15−18. doi: 10.3969/j.issn.1005-9350.2016.06.004
[22] 宋心远. 活性染料染色[M]. 北京: 中国纺织出版社, 2009. Song X Y. Reactive dyeing[M]. Beijing: China Textile Press, 2009.
[23] 朱振旭. 活性染料在非极性介质中染色及机理研究[D]. 杭州: 浙江理工大学, 2017. Zhu Z X. Study on dyeing and mechanism of reactive dyes in non-polar medium[D]. Hangzhou: Zhejiang University of Science and Technology, 2017.
[24] 吕东军. O-羧甲基壳聚糖支载色素和负载催化剂的合成和表征[D]. 天津: 天津大学, 2017. Lü D J. Synthesis and characterization of O-carboxymethyl chitosan grafted colorants and supported catalysts[D]. Tianjin: Tianjin University, 2017.
-
期刊类型引用(3)
1. 陈彩云. 天然染料在鲜茧丝染色中的应用. 轻纺工业与技术. 2023(02): 145-147 .  百度学术
百度学术
2. 杨雨桐,张卿硕,符韵林,孙静. 巴里黄檀心材色素的提取及其抑菌活性. 东北林业大学学报. 2020(10): 100-103+119 .  百度学术
百度学术
3. 王敬贤. 木材染色技术研究进展. 林业科技. 2020(06): 42-47 .  百度学术
百度学术
其他类型引用(4)



 下载:
下载: