Effects of Crucibulum laeve inoculation on photosynthesis of Salix viminalis cultivated in PAHs-contaminated soil
-
摘要:目的 虽然已有研究表明植物-白腐真菌联合修复是一种更高效的多环芳烃(PAHs)污染土壤修复策略,但由于该策略的作用机理尚不清楚,其在土壤修复实践中的应用前景仍存有疑问。光合作用不仅是植物生长发育的基础,也影响根系分泌物的释放水平,进而影响根际微生物的生长和土壤中PAHs的降解。基于此,我们研究了PAHs污染土壤中接种白腐真菌对植物光合作用的影响。方法 在温室中设置了PAHs污染土壤的盆栽修复实验,以蒿柳做为植物修复材料,选取光合色素含量、气体交换参数的光响应曲线和叶绿素荧光参数做为蒿柳的光合生理指标,探究了PAHs污染土壤中接种白腐真菌对蒿柳光合作用的影响。结果 PAHs污染土壤中接种白腐真菌对蒿柳光合色素含量、净光合速率(Pn)、蒸腾速率(Tr)、最大光化学效率(Fv/Fm)、光系统Ⅱ(PSⅡ)潜在活性(Fv/F0)等指标均有积极的促进作用,但降低了叶片的气孔导度(Gs)、胞间CO2浓度(Ci)、非光化学淬灭(NPQ)和光化学淬灭(qP)参数。不同的接种方法能改变Gs、Tr的光响应曲线的变化趋势。此外蒿柳-白腐真菌联合修复显著提高了土壤菲(PHE)、芘(PYR)的去除率。结论 PAHs污染土壤中,白腐真菌能提高蒿柳的光合能力和蒸腾效率,促进土壤PHE和PYR的去除,这对揭示植物−白腐真菌联合修复的作用机理有重要意义。Abstract:Objective Although the study has shown that plant-white rot fungi (WRF) combined remediation is a more efficient remediation strategy for PAHs-contaminated soil, the mechanism of this strategy is still unclear, and its application prospects remain questionable. Photosynthesis is the basis of plant growth and development, and it affects the release level of root exudates, which in turn alters the growth of rhizosphere microorganisms and the degradation of soil-borne PAHs. Based on this, for the first time, this study reported the effects of WRF inoculation on photosynthesis of Salix viminalis cultivated in PAHs-contaminated soil.Method In this study, a pot experiment was conducted in greenhouse for bioremediation of PAHs-contaminated soils and S. viminalis was used as phytoremediation materials. Photosynthetic pigment content, light response curve of gas exchange parameters and chlorophyll fluorescence parameters were selected as the photosynthetic physiological indexes of S. viminalis and the effects of WRF inoculation on the photosynthesis of S. viminalis were reported.Result Results showed that the inoculated WRF positively promoted photosynthetic pigment content, net photosynthetic rate (Pn) and transpiration rate (Tr), maximum photochemical efficiency (Fv/Fm), potential activity of PSII (Fv/F0) of S. viminalis, but reduced their stomatal conductance (Gs) and intercellular CO2 concentrations (Ci), non-photochemical quenching (NPQ) and photochemical quenching (qP). Different inoculation methods changed variation trend of light response curve of Gs and Tr. Besides, the combination of S. viminalis and WRF significantly increased the removal rate of soil-borne phenanthrene and pyrene.Conclusion In this study, WRF increases the photosynthetic rate and transpiration efficiency of S. viminalis, enhances the removal of soil-borne PHE and PYR. In general, our study is significant to reveal the mechanism of plant-WRF combined remediation.
-
Keywords:
- Salix viminalis /
- white-rot fungi (WRF) /
- photosynthesis /
- PAHs-contaminated soil
-
多环芳烃(polycyclic aromatic hydrocarbons,PAHs)是一类具有致癌、致畸、致突变效应的有机污染物,可在水体、土壤或沉积物中积累,进入食物链中并通过富集作用对人类健康产生极大威胁[1-2],这些事实促使研究人员想方设法从自然环境中清除它们。在PAHs污染修复的诸多方法中,生物修复具有绿色环保、成本低、可大面积应用等优点[3-4],是目前最具潜力的修复方法之一。一般来说,PAHs的环数越高,其化学结构越稳定、疏水性越高,利用生物吸收和降解越是困难[5-7]。白腐真菌是PAHs降解真菌,其分泌的木质素酶是胞外酶且底物专一性低,能在土壤环境中广泛分散并通过氧化作用催化自由基的形成,使PAHs的共价键不稳定,从而提高各环数PAHs的生物可利用性[8-9]。然而诸多报道提及了利用白腐真菌原位降解PAHs未能达到控制实验的效果[10-13]。近来García-Sánchez等人[4]研究发现,在玉米(Zea mays)的根际污染土壤中接种平滑白蛋巢菌(Crucibulum laeve,一种白腐真菌)能显著提高土壤PAHs的降解率,但是由于植物−白腐真菌联合修复的作用机理尚不清楚,该方法在土壤修复实践中的应用前景仍存有疑问。
植物从土壤中吸收和提取PAHs的能力有限,但它们能促进根际土壤环境中PAHs的降解,这是植物和根际微生物相互协作的结果[14]。据报道,植物利用约20%的光合产物合成有机分子并以根系分泌物的形式释放到土壤中[15]。高碳释放量有利于提高包括PAHs降解菌在内的根际微生物的生长,高碳释放量植物对PAHs降解菌的富集能力更高[16]。光合作用不仅是植物生长发育的基础,还影响根系分泌物的释放水平,进而影响根际微生物的生长和土壤中PAHs的降解。通常木本植物生物量大、根系发达,其碳释放量(35%)要高于多数植物的平均水平[16],因而是植物和降解菌联合修复策略中更加理想的植物选择。木本植物蒿柳(Salix viminalis)对污染物的富集或去除能力强,其生长迅速、轮伐期短、矮林作业容易,在重金属和有机污染物等各类污染的植物修复领域已有研究和应用[17-22]。研究表明,盆栽实验中蒿柳能强力去除土壤中的菲、蒽、荧蒽、芘等多种PAHs [23-24]。基于此,本研究以蒿柳为研究对象,采用两种不同的接种方式将白腐真菌接种于栽有蒿柳的PAHs污染土壤中,选取光合色素含量、气体交换参数的光响应曲线和叶绿素荧光参数做为蒿柳的光合生理指标,首次研究了PAHs污染土壤中接种白腐真菌对蒿柳光合作用的影响,以期为植物−白腐真菌联合修复的作用机理研究,进而为开拓植物和白腐真菌在PAHs污染土壤生物修复中的应用提供理论依据。
1. 材料和方法
1.1 土壤的采集及人工污染
实验土壤取自北京市昌平区北京市农林科学院实验基地(40°10′59″ N、116°27′21″ E),在土层0 ~ 20 cm深度范围内取样。该地土壤是中国北方常见的黄褐色土,土壤质地为中黏土。所取土壤于实验室室温下晾干,过2 mm网筛。土壤的部分理化性质如下:pH 8.7,阳离子交换量 23.1 cmol/kg,有机质 20.1 g/kg,总氮 972.0 mg/kg,菲(PHE)36.6 μg/kg,芘(PYR)17.4 μg/kg,苯并(a)芘(BaP)28.5 μg/kg,总PAHs 280.3 μg/kg。往10%土壤中加入高纯度的PHE、PYR、BaP(溶于丙酮,分别按4 000、4 000、2 000 μg/kg的含量,土壤PAHs背景值较低可忽略)混合液并置于通风柜中,待丙酮完全挥发后,将污染土壤和剩余90%无污染土壤均匀混合,装入塑料箱中置于室温下老化6周,再次过2 mm网筛并测定PAHs含量。老化后的土壤随机取3份样品,测得PHE、PYR、BaP含量分别为(2 200.2 ± 41.1)μg/kg、(2 643.7 ± 61.7)μg/kg、(1 386.8 ± 41.5)μg/kg。
1.2 植物育苗和真菌接种物制备
剪取种植于中国林业科学研究院苗圃的同株蒿柳(引种自中国科学院沈阳生态所植物园)的1年生枝条,按直径(1.0 ± 0.2)cm的规格,在水中剪成12 cm长插条,随后将插条插入清水中吸足水分,将插条扦插于营养钵中。栽培基质的草炭土:珍珠岩为6∶1(v/v),定时浇水。发芽后在温室中继续培养约5周,选择长势良好,株高均值(18.52 ± 1.16)cm,地径均值(1.81 ± 0.11)mm的蒿柳扦插苗用于实验。
本研究使用的白腐真菌为平滑白蛋巢菌,基于研究报道其在PAHs污染土壤中定殖能力强、生长繁殖迅速[4,25]。真菌菌种购自中国普通微生物菌种保藏管理中心(北京),所得菌种接种到麦芽提取物培养基上,24 ℃下静置培养2周获取新鲜的接种物。按照Reina等人[26]描述的方法进行固态发酵并做修改。利用大麦(Hordeum vulgare)种子做为白腐真菌的纤维素基质载体,在1 000 mL锥形瓶中加72 g大麦种子和120 mL无菌水,高压灭菌,随后将4个真菌琼脂板在80 mL无菌水(55% ,v/w)中匀浆,取40 mL接种到大麦培养基,在24 ℃下静置培养4周,获取真菌接种物。
1.3 盆栽实验
PAHs污染土壤修复实验在中国林业科学研究院温室中进行,温室温度20 ~ 28 ℃,14 h/10 h的光/暗周期。取一系列完全相同的聚丙烯盆,总容积为5 L,每盆中单独堆放约5 kg污染土壤,设置4种修复策略。
NA:自然衰减(不额外引入生物,利用土壤自身的净化能力去除PAHs);
M:真菌强化(将大麦种子混入土壤中,接种0.05 g/盆平滑白蛋巢菌接种物);
P:植物修复(土壤中种植蒿柳);
PMR:植物−真菌联合修复(土壤中种植蒿柳,并接种白腐真菌)。
PMR的实施过程中使用两种真菌接种方法:
PMR1:将大麦种子混入土壤中并移栽蒿柳,接种0.05 g/盆平滑白蛋巢菌接种物;
PMR2:将真菌接种物直接混入土壤的同时移栽蒿柳。
应用于污染土壤的真菌接种物或大麦种子剂量为0.6:10(w/w)。土壤水分保持在60%的田间持水量,需要定期称量,并添加蒸馏水。实验每周随机改变聚丙烯盆位置1次,定期观察记录蒿柳的生长情况,在实验第30天用螺旋状取土钻从各处理的土壤剖面中抽取5个小样本,将小样本的土壤混合后过2 mm网筛,低温干燥后对土壤PAHs含量进行测定,每个处理均有3个重复。此时P与PMR处理的土壤PHE、PYR、BaP含量呈现显著差异(P < 0.05),选取植物叶片进行光合生理指标的测定。
1.4 土壤PAH含量的定量分析
PAH含量的分析参照García-Sánchez等人[4]的方法并做修改。首先对15 g土壤样品用超声波提取法提取,将提取液与去离子水混合,并在相分离后用Sep-Pak硅胶柱清洁并通过旋转真空蒸发浓缩。随后使用Agilent HP 7890 气相色谱仪对PHE、PYR、BaP的含量进行检测,该色谱仪连接到Agilent HP 5975 C惰性质量选择检测器(GC/MSD,7890/ 5975 C,Agilent Technologies,美国)。检测方法参照Fellet等人[27]的报道。所有PAHs的含量均基于土壤干质量呈现。
1.5 光合色素含量
随机选取4株蒿柳的中上部叶,去主脉后剪成0.3 cm × 0.3 cm大小。称0.2 g叶片置于试管中,用80%的丙酮溶液定容至20 mL,黑暗浸提48 h后,测定663、645和470 nm处的吸光度,依照Arnon[28]的方法计算叶绿素a、叶绿素b、类胡萝卜素和总光合色素的含量。
1.6 气体交换参数的光响应曲线绘制
每个处理随机选取4株蒿柳的中上部叶片,选择在晴朗天的09:00—11:00,用便携式光合测定仪Li-Cor 6400进行气体交换参数测定。测定前将植株转移到光下活化 20 min,调节叶室温度为26 ℃,CO2浓度设置为环境CO2浓度。将光合有效辐射(PAR)梯度由高到低设置为 2 000、1 500、1 200、960、840、720、600、480、360、240、120、0 μmol/(m2∙s)共12个梯度,测定不同PAR所对应的净光合速率(Pn)、气孔导度(Gs)、胞间二氧化碳浓度(Ci)和蒸腾速率(Tr),叶室内叶片长度为 3 cm,叶室两头及中间叶宽用直尺测定,叶片面积按两个梯形计算。每个梯度下控制测定时间为180 s,绘制各气体交换参数的光响应曲线(Pn - PAR曲线等)。
1.7 叶绿素荧光参数测定
使用Fluor Pen FP 100手持式叶绿素荧光仪(北京澳作生态仪器有限公司)在日落后(19:00—21:00)叶片暗适应30 min后测定蒿柳中上部叶片,读取最大光化学效率(Fv/Fm)、PSⅡ潜在活性(Fv/F0)、非光化学淬灭系数(NPQ)和光化学淬灭系数(qP)等叶绿素荧光参数,每个处理均检测4个叶片。
1.8 统计分析
各处理中测定的土壤PAHs含量和蒿柳的光合生理指标数据均采用SPSS 18.0软件对各指标数据进行单因素方差分析(ANOVA)和Tukey检验(P < 0.05)。用Origin 8.5进行绘图。
2. 结果与分析
2.1 各PAHs污染土壤修复处理对土壤PAHs含量的影响
由表1可知,各处理下土壤PHE、PYR、BaP含量较初始含量均明显降低(P < 0.05),其中PMR1、PMR2处理下土壤PHE含量分别降低了51.5%、49.2%,明显高于P处理的16.5%(P < 0.05)。PMR1、PMR2处理下土壤PYR含量分别降低了44.6%、51.3%,明显高于P处理的9.9%(P < 0.05)。PMR1和PMR2处理下土壤BaP含量分别降低了38.9%、23.5%,明显高于P处理的15.5%(P < 0.05)。
表 1 各生物修复处理对土壤PAHs含量的影响(第30天)Table 1. Effects of bioremediation treatments on concentration of soil-borne PAHs (on 30th day)μg/kg PAHs含量
PAHs concentrationNA处理
NA treatmentM处理
M treatmentP处理
P treatmentPMR1处理
PMR1 treatmentPMR2处理
PMR2 treatment菲 Phenanthrene 1 602.53 ± 3.27b 1 341.47 ± 28.80c 1 836.57 ± 30.51a 1 067.60 ± 30.60d 1 117.10 ± 33.78d 芘 Pyrene 2 209.50 ± 57.24b 1 626.03 ± 26.30c 2 382.87 ± 53.72a 1 463.50 ± 46.54d 1 288.63 ± 18.74e 苯并[a]芘 Benzo[a]pyrene 1 385.13 ± 37.76a 882.10 ± 11.85c 1 249.27 ± 35.09ab 847.50 ± 28.79c 1 061.40 ± 22.29b 注:每个处理由3个重复计算平均值(± 标准差),不同字母表示用Tukey检验在P < 0.05的水平上有显著差异。NA为自然衰减;M为真菌强化;P为植物修复;PMR1和PMR2均为植物−微生物联合修复。Notes: means (± SD) are calculated from three replications (n=3) for each treatment. Different letters mean significantly different at P < 0.05 level applying Tukey test. NA, natural attenuation; M, mycoaugmentation; P, phytoremediation; PMR1 and PMR2, plant-microbial remediation. 2.2 各处理下蒿柳光合色素含量比较
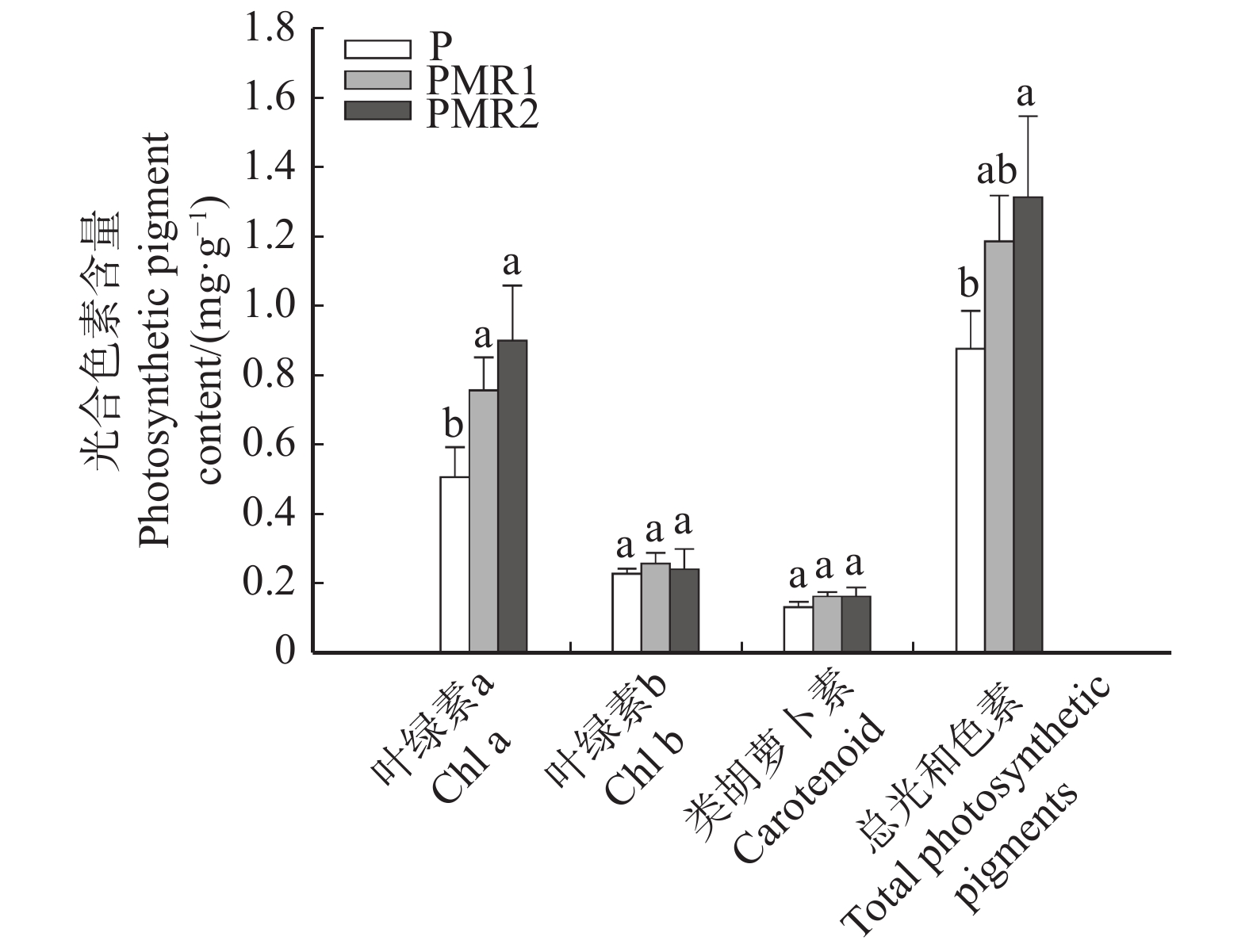
由图1可知,PMR1、PMR2处理下的蒿柳较P处理叶绿素a的含量显著增加(P < 0.05),分别增加了48.9%和76.8%,叶绿素b和类胡萝卜素含量略有增加但差异不显著;PMR1与PMR2处理的蒿柳3种色素含量差异均不显著。总叶绿素含量方面,PMR2相比P处理显著增加了蒿柳的总光合色素含量(P < 0.05),增幅为49.6%;PMR1较P处理增加了蒿柳的总光合色素含量(35.2%)但差异不显著。
2.3 各处理下蒿柳气体交换参数的光响应曲线比较
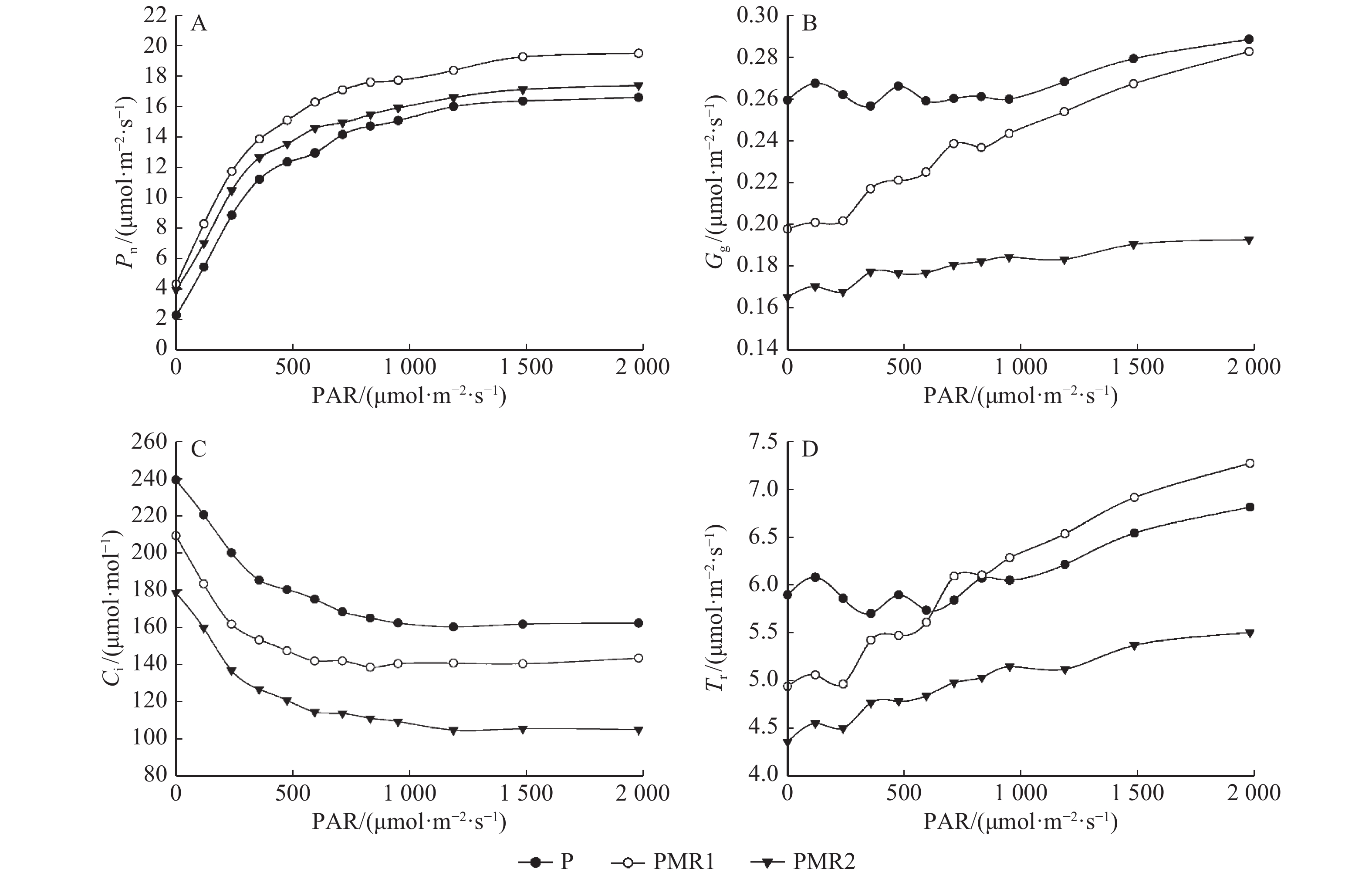
随着PAR的升高,3种处理下蒿柳的Pn呈现相似的变化趋势:0 ~ 480 μmol/(m2∙s),Pn急剧上升,大于480 μmol/(m2∙s)后,Pn上升趋势放缓,几乎平缓。在整个PAR升高的过程中,蒿柳的Pn均为PMR1 > PMR2 > P(图2A)。3种处理下蒿柳的Gs都会随着PAR的升高而增强,且P > PMR1 > PMR2,其中P和PMR2呈现相似的上升趋势,PMR1处理下的Gs在PAR大于480 μmol/(m2∙s)后上升趋势明显快于其他处理,且不断缩小与P处理的差距直至与P处理几乎相等(图2B)。图2C表明,各处理下蒿柳的Ci的变化趋势相似,均为先急剧下降后逐渐放缓,直至平缓,且P > PMR1 > PMR2。蒸腾速率方面(图2D),随着PAR的升高,3种处理下叶片的Tr均呈现总体上升趋势,P和PMR1的Tr高于PMR2;PMR1处理相比其他两种处理的Tr上升趋势明显更快,当PAR低于600 μmol/(m2∙s)时PMR1 < P,PAR高于600 μmol/(m2∙s)之后PMR1 > P。综上,PAHs污染土壤中接种白腐真菌提高了蒿柳的Pn,但降低了Gs和Ci,不同的真菌接种方式对Gs、Tr等参数的变化趋势影响明显。
2.4 各处理下蒿柳叶绿素荧光参数比较
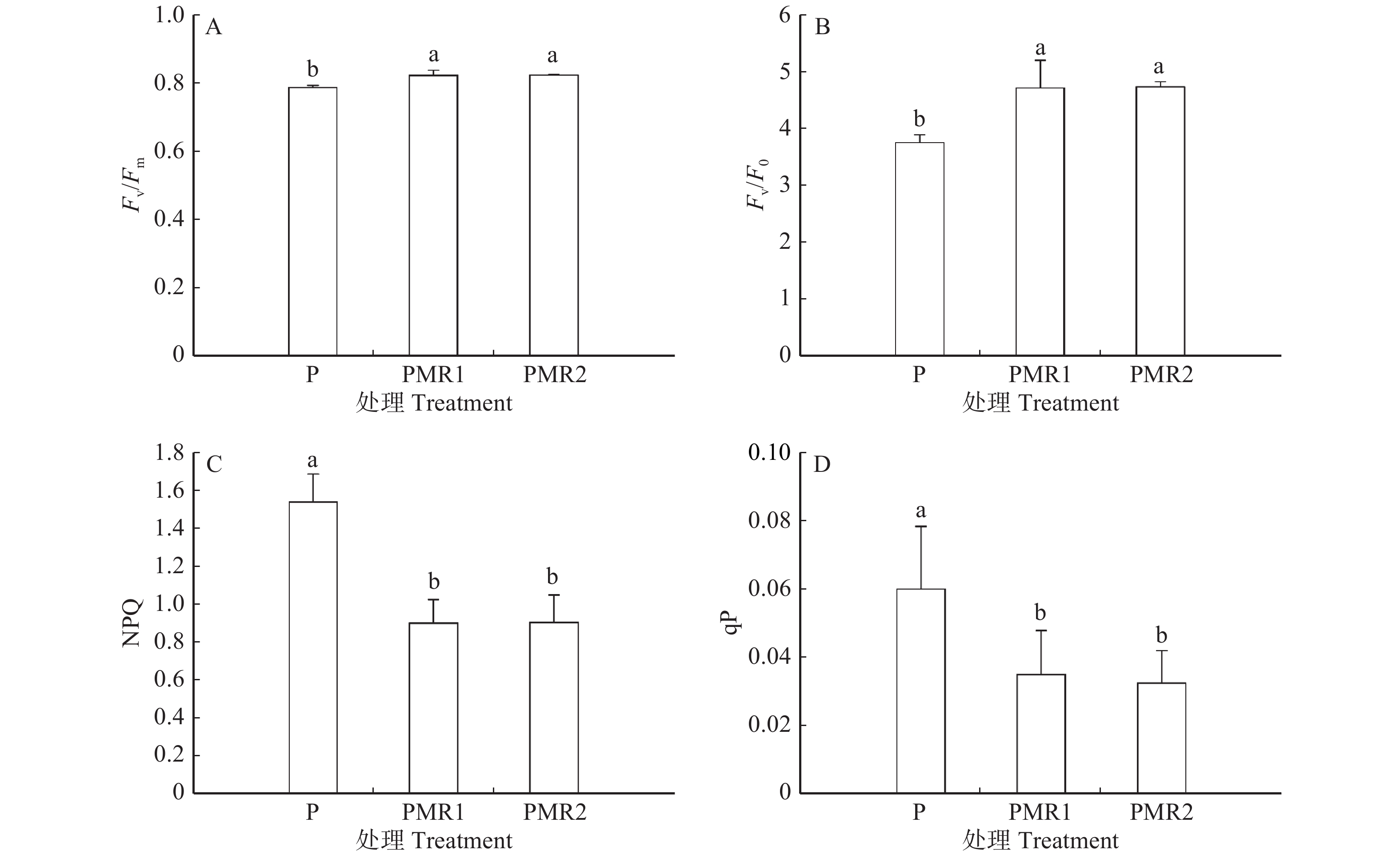
实验30 d后,PMR1与PMR2处理下蒿柳的4个叶绿素荧光参数均无显著性差异,不同的真菌接种方式对蒿柳叶绿素荧光参数影响不大(图3)。但接种白腐真菌的蒿柳叶片的4个叶绿素荧光参数均有明显变化(P < 0.05)。PMR处理显著提高了蒿柳的Fv/Fm(PMR1提高4.4%,PMR2提高4.6%)和Fv/F0(PMR1提高25.4%,PMR2提高25.9%),但降低了NPQ(PMR1降低41.3%,PMR2降低41.1%)和qP(PMR1降低41.7%,PMR2降低45.8%)。
3. 讨 论
本研究采用两种方法接种于PAHs污染土壤中的平滑白蛋巢菌,均促进了蒿柳的土壤PHE和PYR去除,该结果与García-Sánchez等人的[4]相同,他们研究中接种的平滑白蛋巢菌显著增强了栽培玉米土壤中PAHs 去除。不同的是,本研究中P处理的土壤PHE和PYR去除率相比NA处理显著减少,这表明短期内蒿柳的快速生长阻碍了土壤中PHE和PYR的去除,然而PMR却是对PHE和PYR去除率最高的处理,这表明了蒿柳和白腐真菌发生了协同作用,而并非两者PAHs去除能力的加和。同时,本研究未发现白腐真菌[25]能促进植物的生长发育,这与菌根真菌等共生真菌对植物生长发育的积极影响[29-30]不同,由此看来,白腐真菌与植物之间并非典型的互利共生的关系。进一步说,不同于菌根真菌通过提高植物的生物量和抗逆能力进而增强对PAHs的适应和去除,植物−白腐真菌联合修复有其独特的作用机理。
光合色素是参与光合作用的重要色素,叶绿素可以收集、传递光能,其中叶绿素a将光能转化为化学能,而类胡萝卜素可通过吸收剩余光能、淬灭单线态氧,防止膜脂过氧化,起保护叶绿素和光合机能的作用。与外生菌根真菌相同[31],白腐真菌接种处理能提高蒿柳的总光合色素含量,其中对叶绿素a含量的提高效果尤为明显,说明白腐真菌可以通过提高蒿柳的光合色素含量来提高光合效率,进一步增加光合作用产物的产出。一般认为,植物受到外界环境的胁迫,其光合色素被破坏和分解,光合作用会受到抑制,表现为各光合色素含量的降低[32],而白腐真菌则加速了植物光合色素的合成,这表明了蒿柳具有应对白腐真菌刺激的独特响应机制。
诸多研究表明接种真菌能增加树种的净光合速率[31-33],本研究中两种方法接种白腐真菌使得蒿柳的净光合速率均有所增加,这印证了白腐真菌提高蒿柳光合色素含量的结果。气孔是植物与外界进行气体交换的重要通道,在PMR1和PMR2处理下,蒿柳的气孔导度和CO2胞间浓度均下降(相比P处理),即PMR处理不能增大蒿柳的气孔导度,但可以提高其胞间CO2同化速率。此外,随着PAR的升高,PMR1处理较其他处理能增大蒿柳的气孔导度变化范围。通常生长势越旺盛的植物蒸腾速率越高,在PAR低于600 μmol/(m2∙s)时各处理下的蒸腾速率的确如此,此时蒸腾速率与株高、地径成正比。然而,当PAR高于600 μmol/(m2∙s)时,PMR1处理下叶片的蒸腾速率超过了P处理,说明PMR能够显著提高蒿柳的蒸腾能力,间接表明白腐真菌能提高蒿柳根系的吸水能力。
叶绿素荧光利用植物体的叶绿素作为天然探针,可快速、灵敏、无损伤的测定多种因子对植物光合作用的影响[34]。本研究利用叶绿素荧光技术测定发现,PMR处理可显著提高蒿柳的Fv/Fm和Fv/F0,说明白腐真菌提高了蒿柳叶片的光合效率,解释了本研究中净光合速率提高的结果。与之相反的是NPQ和qP的显著降低,NPQ的降低预示着PMR处理下蒿柳叶片通过热耗散减轻过剩光能伤害的能力降低,因此更易受强光的伤害,qP的降低则说明了叶片PSⅡ反映中心开放程度的下降和电子传递活性的降低[35],这表明接种白腐真菌对植物亦有不利影响。
4. 结论与展望
本研究重点探讨了PAHs污染土壤中接种白腐真菌对蒿柳光合作用的影响,结果显示白腐真菌接种处理对蒿柳叶片光合色素含量、Fv/Fm、Fv/F0、Pn、Tr等均有积极的促进作用,对NPQ、qP、Gs、Ci有抑制作用,不同的接种方法会影响Gs和Tr光响应曲线的变化趋势。总的看来,白腐真菌促进了蒿柳的光合能力、蒸腾速率和土壤PHE和PYR的降解。根据García-Sánchez等人[4]的观点,白腐真菌对玉米的刺激作用会改变其根系分泌物,从而影响PAHs的生物可利用性。由此推测,白腐真菌通过促进植物的光合作用,可能使植物增加了根系分泌物的释放,这对于促进根际微生物的生长和PAHs的降解非常重要。因此,今后还需对接种与非接种白腐真菌的蒿柳根系分泌物进行定性、定量的比较研究,并结合土壤微生物区系的变化,对植物−白腐真菌联合修复的作用机理进行更深层次的剖析。
-
表 1 各生物修复处理对土壤PAHs含量的影响(第30天)
Table 1 Effects of bioremediation treatments on concentration of soil-borne PAHs (on 30th day)
μg/kg PAHs含量
PAHs concentrationNA处理
NA treatmentM处理
M treatmentP处理
P treatmentPMR1处理
PMR1 treatmentPMR2处理
PMR2 treatment菲 Phenanthrene 1 602.53 ± 3.27b 1 341.47 ± 28.80c 1 836.57 ± 30.51a 1 067.60 ± 30.60d 1 117.10 ± 33.78d 芘 Pyrene 2 209.50 ± 57.24b 1 626.03 ± 26.30c 2 382.87 ± 53.72a 1 463.50 ± 46.54d 1 288.63 ± 18.74e 苯并[a]芘 Benzo[a]pyrene 1 385.13 ± 37.76a 882.10 ± 11.85c 1 249.27 ± 35.09ab 847.50 ± 28.79c 1 061.40 ± 22.29b 注:每个处理由3个重复计算平均值(± 标准差),不同字母表示用Tukey检验在P < 0.05的水平上有显著差异。NA为自然衰减;M为真菌强化;P为植物修复;PMR1和PMR2均为植物−微生物联合修复。Notes: means (± SD) are calculated from three replications (n=3) for each treatment. Different letters mean significantly different at P < 0.05 level applying Tukey test. NA, natural attenuation; M, mycoaugmentation; P, phytoremediation; PMR1 and PMR2, plant-microbial remediation. -
[1] Buonanno G, Giovinco G, Morawska L, et al. Lung cancer risk of airborne particles for Italian population[J]. Environmental Research, 2015, 142: 443−451. doi: 10.1016/j.envres.2015.07.019
[2] Joner E J, Leyval C, Colpaert J V. Ectomycorrhizas impede phytoremediation of polycyclic aromatic hydrocarbons (PAHs) both within and beyond the rhizosphere[J]. Environmental Pollution, 2006, 142(1): 34−38. doi: 10.1016/j.envpol.2005.09.007
[3] Agnello A C, Bagard M, Van Hullebusch E D, et al. Comparative bioremediation of heavy metals and petroleum hydrocarbons co-contaminated soil by natural attenuation, phytoremediation, bioaugmentation and bioaugmentation-assisted phytoremediation[J]. Science of the Total Environment, 2016, 563−564: 693−703. doi: 10.1016/j.scitotenv.2015.10.061
[4] García-Sánchez M, Kosnar Z, Mercl F, et al. A comparative study to evaluate natural attenuation, mycoaugmentation, phytoremediation, and microbial-assisted phytoremediation strategies for the bioremediation of an aged PAH-polluted soil[J]. Ecotoxicology and Environmental Safety, 2018, 147: 165−174. doi: 10.1016/j.ecoenv.2017.08.012
[5] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures[C]. Lyons: International Agency for Research on Cancer, 2010.
[6] Bamforth S M, Singleton I. Bioremediation of polycyclic aromatic hydrocarbons: current knowledge and future directions[J]. Journal of Chemical Technology & Biotechnology, 2005, 80(7): 723−736.
[7] Ghosal D, Ghosh S, Dutta T K, et al. Current state of knowledge in microbial degradation of polycyclic aromatic hydrocarbons (PAHs): a review[J/OL]. Frontiers in Microbiology, 2016, 7: 1369 (2016−11−15) [2018−08−12]. https://doi.org/10.3389/fmicb.2016.01837.
[8] Lladó S, Gràcia E, Solanas A M, et al. Fungal and bacterial microbial community assessment during bioremediation assays in an aged creosote-polluted soil[J]. Soil Biology and Biochemistry, 2013, 67: 114−123. doi: 10.1016/j.soilbio.2013.08.010
[9] Haritash A K, Kaushik C P. Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review[J]. Journal of Hazardous Materials, 2009, 169(1−3): 1−15. doi: 10.1016/j.jhazmat.2009.03.137
[10] Radtke C, Cook W S, Anderson A. Factors affecting antagonism of the growth of Phanerochaete chrysosporium by bacteria isolated from soils[J]. Applied Microbiology & Biotechnology, 1994, 41(2): 274−280.
[11] Wiesche C I D, Martens R, Zadrazil F. The effect of interaction between white-rot fungi and indigenous microorganisms on degradation of polycyclic aromatic hydrocarbons in soil[J]. Water Air & Soil Pollution, 2003, 3(3): 73−79.
[12] Borràs E, Caminal G, Sarrà M, et al. Effect of soil bacteria on the ability of polycyclic aromatic hydrocarbons (PAHs) removal by Trametes versicolor and Irpex lacteus from contaminated soil[J]. Soil Biology and Biochemistry, 2010, 42(12): 2087−2093. doi: 10.1016/j.soilbio.2010.08.003
[13] Gao D, Du L, Yang J, et al. A critical review of the application of white rot fungus to environmental pollution control[J]. Critical Reviews in Biotechnology, 2010, 30(1): 70−77. doi: 10.3109/07388550903427272
[14] Alagić S Č, Maluckov B S, Radojičić V B. How can plants manage polycyclic aromatic hydrocarbons? May these effects represent a useful tool for an effective soil remediation? A review[J]. Clean Technologies and Environmental Policy, 2014, 17(3): 597−614.
[15] Huang A C, Jiang T, Liu Y X, et al. A specialized metabolic network selectively modulates Arabidopsis root microbiota[J/OL]. Science, 2019, 364(2019−05−10)[2019−07−25]. https://doi.org/10.1126/science.aau6389.
[16] Marmiroli M, Pietrini F, Maestri E, et al. Growth, physiological and molecular traits in Salicaceae trees investigated for phytoremediation of heavy metals and organics[J]. Tree Physiology, 2011, 31(12): 1319−1334. doi: 10.1093/treephys/tpr090
[17] Berndes G, Fredrikson F, Börjesson P. Cadmium accumulation and Salix-based phytoextraction on arable land in Sweden[J]. Agriculture, Ecosystems & Environment, 2004, 103(1): 207−223.
[18] Ucisik A S, Trapp S. Uptake, removal, accumulation, and phytotoxicity of 4-chlorophenol in willow trees[J]. Archives of Environmental Contamination and Toxicology, 2008, 54(4): 619−627. doi: 10.1007/s00244-007-9065-6
[19] Oleszczuk P, Baran S. Polycyclic aromatic hydrocarbons content in shoots and leaves of willow (Salix viminalis) cultivated on the sewage sludge-amended soil[J]. Water, Air, and Soil Pollution, 2005, 168(1−4): 91−111. doi: 10.1007/s11270-005-0884-7
[20] Oleszczuk P, Godlewska P, Reible D D, et al. Bioaccessibility of polycyclic aromatic hydrocarbons in activated carbon or biochar amended vegetated (Salix viminalis) soil[J]. Environmental Pollution, 2017, 227: 406−413. doi: 10.1016/j.envpol.2017.04.064
[21] Bissonnette L, St-Arnaud M, Labrecque M. Phytoextraction of heavy metals by two Salicaceae clones in symbiosis with arbuscular mycorrhizal fungi during the second year of a field trial[J]. Plant and Soil, 2010, 332(1−2): 55−67. doi: 10.1007/s11104-009-0273-x
[22] Shimmen T, Nishikawa S I. Phytoremediation of polycyclic aromatic hydrocarbons in manufactured gas plant-impacted soil[J]. Journal of Environmental Quality, 2005, 34(5): 1755−1762. doi: 10.2134/jeq2004.0399
[23] Önneby K. Phytoremediation of a highly creosote-contaminated soil by means of Salix viminalis[D]. Uppsala : Swedish University of Agricultural Sciences, 2005.
[24] Hultgren J, Pizzul L, Castillo M D P. Degradation of PAH in a creosote-contaminated soil: a comparison between the effects of willows (Salix viminalis), wheat straw and a nonionic surfactant[J]. International Journal of Phytoremediation, 2009, 12(1): 54−66. doi: 10.1080/15226510902767122
[25] Tornberg K, Bååth E, Olsson S. Fungal growth and effects of different wood decomposing fungi on the indigenous bacterial community of polluted and unpolluted soils[J]. Biology and Fertility of Soils, 2003, 37: 190−197. doi: 10.1007/s00374-002-0574-1
[26] Reina R, Liers C, Ocampo J A, et al. Solid state fermentation of olive mill residues by wood- and dung-dwelling Agaricomycetes: effects on peroxidase production, biomass development and phenol phytotoxicity[J]. Chemosphere, 2013, 93(7): 1406−1412. doi: 10.1016/j.chemosphere.2013.07.006
[27] Fellet G, Pošćić F, Licen S, et al. PAHs accumulation on leaves of six evergreen urban shrubs: a field experiment[J]. Atmospheric Pollution Research, 2016, 7(5): 915−924. doi: 10.1016/j.apr.2016.05.007
[28] Arnon D I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris[J]. Plant Physiology, 1949, 24(1): 1−15. doi: 10.1104/pp.24.1.1
[29] Khan Z, Roman D, Kintz T, et al. Degradation, phytoprotection and phytoremediation of phenanthrene by endophyte Pseudomonas putida, PD1[J]. Environmental Science and Technology, 2014, 48(20): 12221−12228. doi: 10.1021/es503880t
[30] Rajtor M, Piotrowska-Seget Z. Prospects for arbuscular mycorrhizal fungi (AMF) to assist in phytoremediation of soil hydrocarbon contaminants[J]. Chemosphere, 2016, 162: 105−116. doi: 10.1016/j.chemosphere.2016.07.071
[31] 宋微, 吴小芹. 外生菌根真菌对‘NL-895杨’光合作用的影响[J]. 西北植物学报, 2011, 31(7):1474−1478. Song W, Wu X Q. Effect of ectomycorrhizal fungi on photosynthesis of poplar NL-895[J]. Acta Bot Boreal-Occident Sin, 2011, 31(7): 1474−1478.
[32] Nxele X, Klein A, Ndimba B K. Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants[J]. South African Journal of Botany, 2017, 108: 261−266. doi: 10.1016/j.sajb.2016.11.003
[33] 朱凌骏, 傅致远, 张金池, 等. 菌根真菌对榉树光合特性的影响[J]. 南京林业大学学报(自然科学版), 2018, 42(6):121−127. Zhu L J, Fu Z Y, Zhang J C, et al. Effects of mycorrhizal fungi on photosynthetic characteristics of Zelkova serrata Thunb[J]. Journal of Nanjing Forestry University (Natural Sciences Edition), 2018, 42(6): 121−127.
[34] Ahammed G J, Wang M M, Zhou Y H, et al. The growth, photosynthesis and antioxidant defense responses of five vegetable crops to phenanthrene stress[J]. Ecotoxicology and Environmental Safety, 2012, 80: 132−139. doi: 10.1016/j.ecoenv.2012.02.015
[35] Bilger W, Björkman O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis[J]. Photosynthesis Research, 1990, 25(3): 173−185. doi: 10.1007/BF00033159



 下载:
下载: