Effects of nitrogen, phosphorus addition and drought on leaf stoichiometry in dominant species of alpine meadow
-
摘要:目的 全球变化背景下,土壤氮和磷有效性及含水量发生显著变化,进而对植物生长和生理过程产生影响。但是,目前同时考虑土壤氮、磷和水分三因素交互作用对植物生长和生理性状的研究还很少,特别是对高寒草甸植物的研究。本研究旨在揭示氮富集、磷富集、干旱及其交互作用对高寒草甸优势植物生长、叶片氮磷含量及其化学计量的影响,为高寒草甸生态系统管理提供科学依据。方法 基于川西北高寒草甸氮添加(10 g/(m 2 ·a))、磷添加(10 g/(m 2·a))与干旱(减雨50%)控制实验,通过测定垂穗披碱草、发草和草玉梅地上生物量、叶片氮含量(N)、磷(P)含量以及N:P比例,分析不同处理及其交互作用对3种植物生物量和叶片养分性状的影响。结果 对于植物生长,氮添加均显著增加3种植物地上生物量,但是磷添加和干旱及不同处理之间的交互作用对植物生物量没有显著影响。对于叶片养分,氮添加显著增加3种植物叶片氮含量和N:P比例,磷添加也增加植物叶片磷含量但降低叶片N:P比例。干旱增加垂穗披碱草与发草的叶片氮含量,对叶片磷含量和N:P比例影响不显著。氮添加与干旱处理之间的交互作用显著增加垂穗披碱草与发草叶片氮含量和N:P比。氮添加与磷添加之间的交互作用对3种植物叶片养分性状没有影响。结论 本研究表明高寒草甸植物生长和养分性状对养分富集、干旱及其交互作的响应格局存在很大差异。氮输入主要影响植物生长,而氮磷养分和干旱及它们之间复杂的交互作用均改变植物养分和化学计量平衡。这些结果指示出未来需要深入研究高寒草甸植物生理过程对全球变化交互作用的响应机理。Abstract:Objective Global change has substantially changed soil nitrogen (N), phosphorus (P) and water availability, which further impacts plant growth and physiological processes. However, so far few studies have been conducted to analyze the interaction effects of soil N, P and water on plant growth and physiological traits, especially for alpine meadow plants. This study aims to reveal the impacts of N addition, P addition, drought and their interactions on plant growth, leaf N and P content and N:P ratios in dominant species of alpine meadows, providing scientific evidence for grassland management.Method Based on an experiment of N addition (10 g/(m2· year)), P addition (10 g/(m2· year)) and drought (50% rainfall reduction) in an alpine meadow of northwestern Sichuan, we measured aboveground biomass, leaf N, P content and their ratio in Elymus nutans, Deschampsia caespitosa and Anemone rivularis. Then we analyzed the influence of different treatments and their interactions on plant biomass and leaf nutrient.Result For plant growth, N addition significantly increased plant biomass of three species, but the impacts of P addition, drought, and the interactions among different treatments were not significant. For leaf nutrient, N addition significantly enhanced leaf N content and N:P ratio of three species, and P addition also promoted leaf P content but reduced N:P ratio. Drought raised leaf N content of E. nutans and D. caespitosa, but had no significant effect on leaf P content and N:P ratio. The interaction of N addition and drought promoted leaf N content and N:P ratio of E. nutans and D. caespitosa. Nevertheless, the interaction of N and P addition was not significant for leaf nutrient of all species.Conclusion This study indicates that alpine species have quite different responses of plant growth versus nutrient traits to nutrient enrichment, drought and their interactions. N input mainly facilitates plant growth, but the complex impacts of soil N, P, drought and their interactions all affect plant nutrient and stoichiometric balance. These results imply that more future studies are needed to detect the mechanisms underlying alpine plant physiological responses to the interactions of various global changes.
-
自工业革命以来,由于人类活动(化石燃料燃烧和农业活动等)导致的大气沉降,已经显著改变陆地生态系统土壤氮和磷有效性[1-2]。同时伴随着全球极端气候频发,降水量格局也发生很大变化,目前干旱已经成为世界性的重大环境问题[3]。上述全球变化因子共同作用会导致土壤氮磷养分和含水量的变化。研究认为土壤氮磷养分输入会缓解植物养分限制[4-6],促进植物生长,同时也会增加植物体内养分含量,从而改变元素之间的化学计量平衡 [[7-8]。氮和磷是植物生长的重要营养元素,在植物能量代谢和新陈代谢等生理过程中起着重要的调节作用[9]。干旱会显著降低土壤水分,而土壤含水量通过影响参与土壤氮磷元素循环转化的酶活性、植物凋落物的分解速率和养分矿化来改变土壤中氮和磷有效性,进而影响植物对土壤养分的吸收[10]。大部分研究表明随着土壤含水量的增加,有利于植物对土壤营养元素的吸收和利用。但也有研究发现,在降水量较多区域,适当的干旱反而增加植物对营养元素的吸收[11-14]。因此,研究植物生长和养分性状对养分富集和土壤水分变化的响应,有利于了解植物对外界环境变化的适应策略和生理过程机理[15-19]。但是,目前同时考虑土壤氮、磷有效性和水分变化对植物生长和养分的研究还很少,特别对于高寒草原植物的研究更少。
全球变化背景下,前人在模拟氮添加、氮磷不同比例添加、增减雨对高寒草甸植物养分含量和化学计量方面进行了大量研究。例如,宾振钧等[20]在青藏高原高寒草甸进行不同梯度氮肥添加实验发现,植物生物量和叶片养分性状受外源氮的输入影响显著,不同物种对氮添加响应的差异导致物种丰富度发生变化,最终影响高寒草甸生态系统植物群落结构[21]。Dong等[22]在青藏高原进行氮磷不同比例添加实验发现,氮磷添加对不同植物功能群影响不同,氮添加显著增加禾本科植物的叶片氮含量,磷添加显著增加杂类草叶片磷含量,而氮磷同时添加对大多数植物叶片的氮磷含量没有显著影响[23-25]。并且,前人在研究植物叶片氮、磷含量及化学计量比对水分变化的响应发现,随着土壤水分减少,植物叶片氮含量有增加[26]、减小[27]和没有变化[28]的不同结果,而植物叶片磷含量均呈现显著降低趋势[26]。因此,土壤水分对植物养分吸收和利用策略影响比较复杂,植物会因不同环境条件产生不同的响应[29-30]。
青藏高原是我国重要生态安全屏障,高寒草甸约占青藏高原草地面积的50%左右。高寒草甸生态系统通常受到土壤氮和磷的限制作用,并对降水变化很敏感。全球变化背景下土壤氮和磷养分、极端干旱以及它们之间复杂的交互作用均可能对高寒草甸植物的生长和化学计量产生显著影响。因此,本研究以青藏高原高寒草甸为研究对象,基于氮添加、磷添加和减雨50%三因素控制实验,选取3种优势植物,通过测定植物生物量和养分,旨在解析养分富集、干旱及其交互作用对高寒草甸植物生长和养分性状的影响机理。
1. 研究区概况
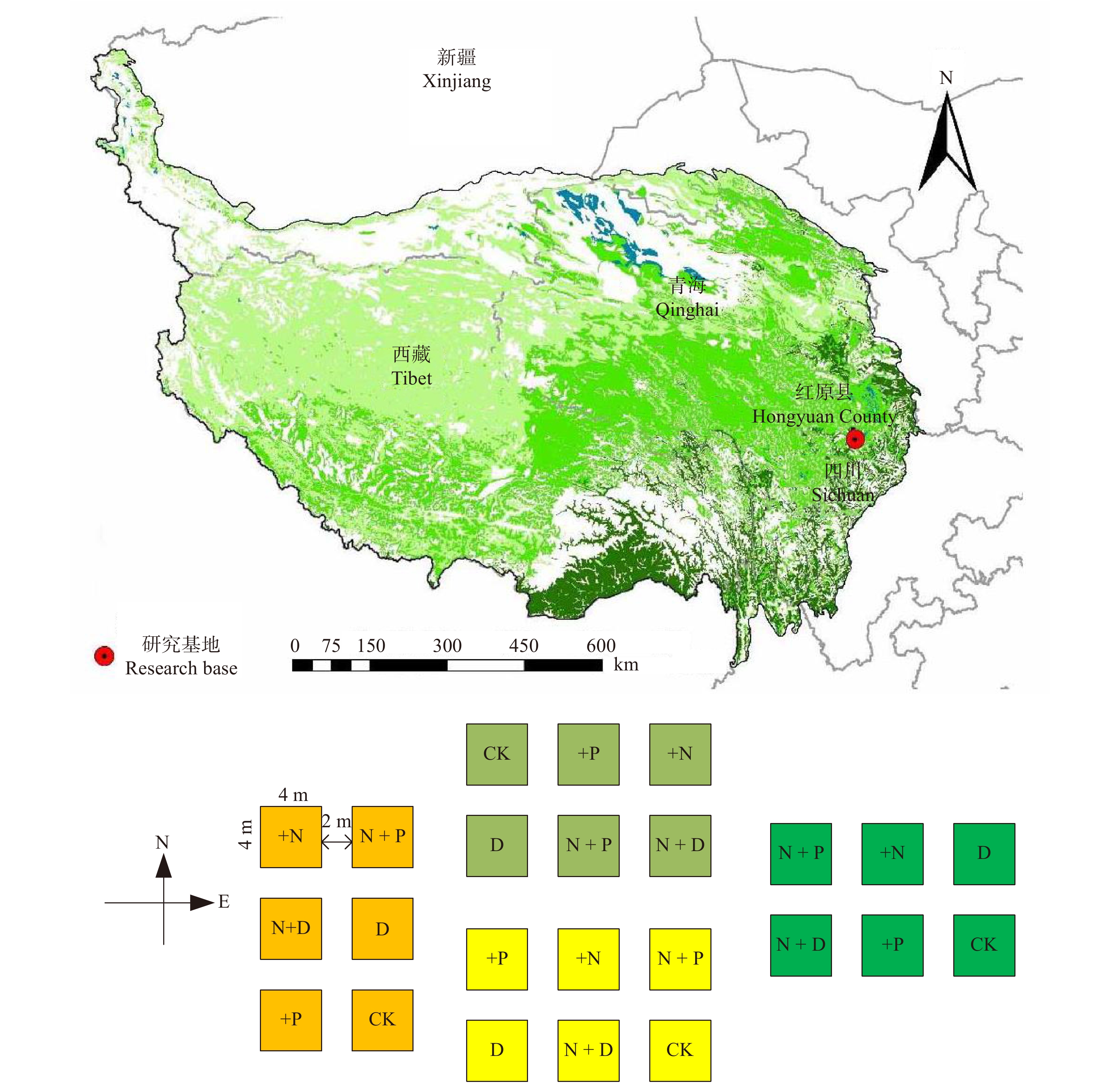
试验样地位于四川省阿坝藏族羌族自治州红原县邛溪镇西南民族大学青藏高原研究基地(32°49′59″N,102°34′53″E,海拔3 490 m)。该地区属于大陆性高原寒温带半湿润季风气候,四季气候分明,春秋季节短暂,冬季漫长寒冷,无夏季。年平均气温1.4 ℃,日温差大。干湿季节分明,年平均降水量650 ~ 800 mm,5—8月降水占全年80%[29]。地带性植被类型为高寒草甸。实验样地是2017年围封的天然草地。植被以禾本科(Gramineae)、莎草科(Cyperaceae)、毛茛科(Ranunculaceae)、菊科(Asteraceae)和豆科(Leguminosae)植物为主,优势植物包括禾本科垂穗披碱草(Elymus nutans)和发草(Deschampsia caespitosa),莎草科薹草(Carex spp.)以及毛茛科草玉梅(Anemone rivularis)等。土壤0 ~ 10 cm有效氮含量为215 mg/kg,有效磷含量为2.875 mg/kg,pH 值5.25。
2. 研究方法
2.1 试验设计
2017年5月,在围栏内选择地势平坦,植被相对均匀的区域,设置包含24个小区的实验样地。每个小区的面积为4 m × 4 m,间隔距离2 m(图1)。实验平台于2018年加入全球大型标准化联网实验Nutrient Network[30]和Drought Network[31],本实验设计与两大全球联网实验设计相同,但同时考虑了养分与干旱之间的交互作用。具体设计为氮添加(10 g/(m2·a))、磷添加(10 g/(m2·a))、减雨50%(干旱)三因子交互控制实验,采取随机区组试验设计。本研究涉及到的实验处理共6个,分别为对照(Control),氮添加(+ N),磷添加(+ P),干旱(D),氮磷同时添加(N + P),氮添加和减雨50%(N + D),每个处理4个重复。氮养分采用树脂包膜尿素(纯N量为46.6%),磷养分采用重过磷酸钙(含P2O5量为12%),减雨50%采用遮雨棚(长3 m × 宽6 m × 高3 m),同时上面搭建树脂透光板实现(透光率大于99%)。施肥时间为每年5月中旬阴雨天,将氮肥和磷肥均匀撒在指定的实验小区内。
![]() 图 1 研究样地位置与小区布置示意图CK. 对照;+N. 氮添加;+P. 磷添加;D. 干旱(减雨50%);N + P. 氮磷同时添加;N + D. 氮添加和减雨50%。下同。CK, control; +N, nitrogen addition; +P, phosphorus addition; D, drought (50% rainfall reduction); N + P, nitrogen and phosphorus adding simultaneously; N + D, nitrogen addition and drought treatment. The same below.Figure 1. Sketch map of the location of research sample plots and layout of experimental plots
图 1 研究样地位置与小区布置示意图CK. 对照;+N. 氮添加;+P. 磷添加;D. 干旱(减雨50%);N + P. 氮磷同时添加;N + D. 氮添加和减雨50%。下同。CK, control; +N, nitrogen addition; +P, phosphorus addition; D, drought (50% rainfall reduction); N + P, nitrogen and phosphorus adding simultaneously; N + D, nitrogen addition and drought treatment. The same below.Figure 1. Sketch map of the location of research sample plots and layout of experimental plots2.2 样品采集、处理与室内测定
本实验选取3种高寒草甸优势植物,分别是垂穗披碱草、发草和草玉梅,其生物量之和占总生物量的50%左右。植物采样时间为2018年8月2日至12日,在每个小区内用两个0.1 m × 1 m样方框剪取所有植物的地上生物量,室内分种并分别装入信封,在65 ℃烘箱中烘48 h(恒质量)后称植物干质量。烘干样品采用球磨仪(NM200,Retsch,Haan,Germany)粉碎,用于测定植物叶片全氮和全磷。全氮含量采用元素分析仪(vario E1 III, Elementar Analysensysteme GmbH, Hanau,Germany)测定,全磷含量测定采用全自动间断化学分析仪(Cleverchem Cleverchem200+,DeChem-Tech GmbH,Germany)测定。同时,我们对每个小区随机选取5个位置,对0 ~ 10 cm土壤进行取样。混匀之后的新鲜土样过2 mm筛并将细根挑干净。土壤样品分成2份,一份在室内风干, 使用球磨仪粉碎,并采用连续流动分析仪(SAN Plus,Skalar,Netherlands)测定土壤可利用氮(SAN),同时使用NaHCO3浸提−钼锑抗比色法测定土壤速效磷(SAP)含量。另外一份土壤样品在冰箱冷冻保存。土壤含水量采用TDR水分仪(TDR350,Aurora,lllinois,USA)测定。
2.3 数据分析
统计分析首先采用三因素方差分析方法(three-way ANOVA)分析各处理及其交互作用对植物地上生物量、叶片氮含量、磷含量及N:P比例的影响。第二,进一步利用T检验比较N + P和N + D处理与对照之间的差异。第三,采用简单线性回归, 分别分析土壤含水量、土壤氮或磷有效性与植物叶片氮、磷含量或叶片N:P比例之间的定量关系。上述统计分析均使用SPSS软件(SPSS 19.0 for windows,SPSS Inc. Chicago,IL,USA)完成, 制图使用Sigma Plot 12.5软件完成。
3. 结果与分析
3.1 不同处理对植物地上生物量的影响
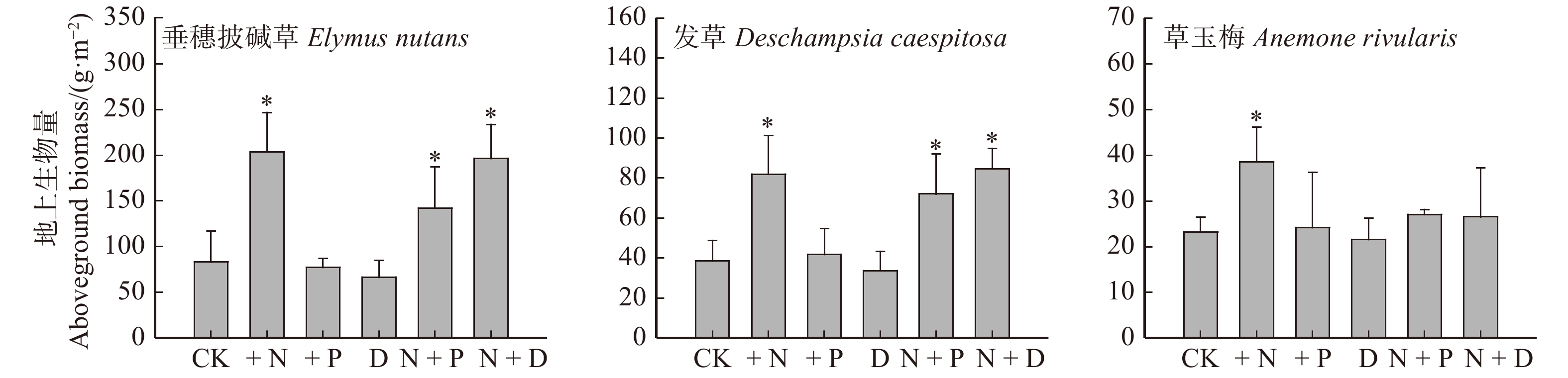
实验结果显示,氮添加显著增加垂穗披碱草、发草和草玉梅的地上生物量,而磷添加、干旱处理对3种植物的生物量影响不显著。与氮添加类似,氮磷同时添加、氮和干旱同时处理均显著增加垂穗披碱草和发草的生物量,但是对草玉梅的影响不显著(图2)。此外,我们没有发现氮添加、磷添加、干旱不同处理之间交互作用对3种植物生物量的显著影响。
![]() 图 2 3种植物地上生物量对氮添加(+ N)、磷添加(+ P)、干旱(D)、氮磷同时添加(N + P)、氮和干旱同时处理(N + D)的响应*表示处理与对照差异显著(P < 0.05)。* indicates a significant difference between treatment and control (P < 0.05).Figure 2. Responses of aboveground biomass in three species to nitrogen addition (+ N), phosphorus addition (+ P), drought (D) , nitrogen and phosphorus adding simultaneously (N + P), and nitrogen addition and drought simultaneously (N + D)
图 2 3种植物地上生物量对氮添加(+ N)、磷添加(+ P)、干旱(D)、氮磷同时添加(N + P)、氮和干旱同时处理(N + D)的响应*表示处理与对照差异显著(P < 0.05)。* indicates a significant difference between treatment and control (P < 0.05).Figure 2. Responses of aboveground biomass in three species to nitrogen addition (+ N), phosphorus addition (+ P), drought (D) , nitrogen and phosphorus adding simultaneously (N + P), and nitrogen addition and drought simultaneously (N + D)3.2 不同处理对植物叶片氮含量、磷含量和N:P比例的影响
实验结果显示,氮添加显著提高垂穗披碱草、发草和草玉梅的叶片氮含量和N:P比例(图3)。类似地,磷添加均增加3种植物叶片磷含量,但降低叶片N:P比例。干旱显著促进垂穗披碱草和发草的叶片氮含量,而对草玉梅叶片氮含量不影响。此外,干旱对3种植物的叶片磷含量和N:P比例都没有处理效应。与氮添加类似,氮磷同时添加、氮与干旱同时处理都显著增加垂穗披碱草和发草的叶片氮含量,而对草玉梅没有影响。对处理间交互作用进一步分析,发现氮添加和干旱对垂穗披碱草和发草的叶片氮含量及N:P比例有正的交互效应,但是对草玉梅没有影响(表1)。氮添加和磷添加对3种植物叶片氮含量,磷含量及N:P值均没有交互影响。
![]() 图 3 3种植物叶片氮、磷含量及N:P比例对氮添加(+ N)、磷添加(+ P)、干旱(D)、氮磷同时添加(N + P)、氮和干旱同时处理(N + D)的响应*表示处理与对照相比差异显著(P < 0.05),黑色柱子表示交互作用显著,虚线表示与交互项比较的2个处理的平均值。* indicates a significant difference between treatment and control (P < 0.05). Black bar indicates a significant interaction effect, and the dashed line indicates the average of the two treatments compared with the interaction item.Figure 3. Responses of leaf nitrogen, phosphorus concentration and N:P ratio in three plant species to nitrogen addition (+ N), phosphorus addition (+ P), drought (D), nitrogen and phosphorus adding simultaneously (N + P), and nitrogen addition and drought simultaneously (N + D)表 1 氮添加(+ N)、磷添加(+ P)、干旱(D)及其交互作用(N × P,N × D)对植物生物量、叶片氮含量、磷含量和N:P比例影响的三因素方差分析Table 1. Three-way ANOVA on the effects of nitrogen addition (+ N), phosphorus addition (+ P), drought (D) and their interactions (N × P, N × D) on plant biomass, leaf nitrogen concentration, phosphorus concentration and N:P ratios
图 3 3种植物叶片氮、磷含量及N:P比例对氮添加(+ N)、磷添加(+ P)、干旱(D)、氮磷同时添加(N + P)、氮和干旱同时处理(N + D)的响应*表示处理与对照相比差异显著(P < 0.05),黑色柱子表示交互作用显著,虚线表示与交互项比较的2个处理的平均值。* indicates a significant difference between treatment and control (P < 0.05). Black bar indicates a significant interaction effect, and the dashed line indicates the average of the two treatments compared with the interaction item.Figure 3. Responses of leaf nitrogen, phosphorus concentration and N:P ratio in three plant species to nitrogen addition (+ N), phosphorus addition (+ P), drought (D), nitrogen and phosphorus adding simultaneously (N + P), and nitrogen addition and drought simultaneously (N + D)表 1 氮添加(+ N)、磷添加(+ P)、干旱(D)及其交互作用(N × P,N × D)对植物生物量、叶片氮含量、磷含量和N:P比例影响的三因素方差分析Table 1. Three-way ANOVA on the effects of nitrogen addition (+ N), phosphorus addition (+ P), drought (D) and their interactions (N × P, N × D) on plant biomass, leaf nitrogen concentration, phosphorus concentration and N:P ratios物种 Species 指标 Index df + N + P D N × P N × D 垂穗披碱草
Elymus nutans生物量 Biomass 1 14.501 1** 1.059 4 0.068 4 0.765 8 0.151 0 氮含量 Nitrogen content 1 17.853 8*** 1.166 6 9.793 2** 1.511 7 4.198 7* 磷含量 Phosphorus content 1 3.550 0 93.236 8*** 0.279 5 0.152 9 1.117 3 N:P 1 31.713 9*** 85.326 3*** 3.112 7 3.829 9 9.121 1** 发草
Deschampsia caespitosa生物量 Biomass 1 54.514 0*** 0.550 5 3.326 9 0.307 1 0.410 3 氮含量 Nitrogen content 1 17.213 7*** 0.037 2 12.443 2** 0.990 2 5.689 5* 磷 含量Phosphorus content 1 0.293 0 26.904 9*** 0.273 2 0.107 6 1.845 0 N:P 1 5.572 1* 69.735 5*** 0.001 9 0.245 9 6.550 4* 草玉梅
Anemone rivularis生物量 Biomass 1 10.190 5* 0.688 7 0.238 8 0.074 9 0.060 0 氮含量 Nitrogen content 1 4.584 5* 2.286 1 1.747 6 0.099 6 0.106 8 磷含量 Phosphorus content 1 2.995 6 9.581 3** 0.268 8 0.053 9 3.304 0 N:P 1 15.580 5*** 24.599 6*** 4.304 1 0.140 3 1.695 2 注:*表示在P < 0.05水平上差异显著;**表示在P < 0.01水平上差异显著;***表示在P < 0.001水平上差异显著。Notes: *, **, *** represent significant difference at P < 0.05, P < 0.01 and P < 0.001 level, respectively. 3.3 水分含量与土壤或植物养分之间关系
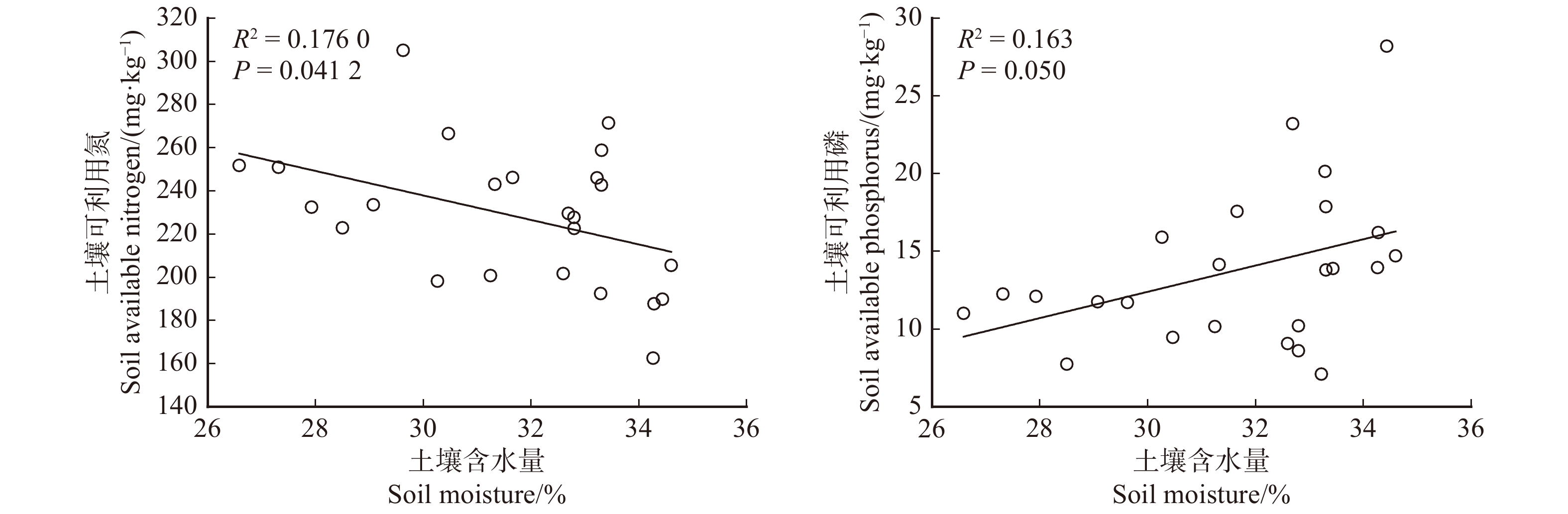
实验结果显示,随土壤含水量的减少,土壤有效氮呈显著增加趋势(图4),但是土壤有效磷呈降低趋势。类似地,我们发现随土壤含水量的降低,垂穗披碱草和发草的叶片氮含量显著升高,而对草玉梅影响不显著。此外,草玉梅叶片磷含量随土壤含水量的降低呈显著降低趋势,而垂穗披碱草叶片N:P比例随土壤含水量的降低显著升高(图5)。
4. 结论与讨论
4.1 讨 论
本研究发现,氮添加增加高寒草甸禾本科植物垂穗披碱草和发草以及杂类草草玉梅的地上生物量,而磷添加和干旱对3种植物的生长影响很小。该结果表明高寒草甸植物生长主要受氮限制,而非磷和水分限制。类似地,传统研究发现在氮限制的生态系统中,氮输入会促进植物地上生物量[32]。此外,宾振钧等[20]在青藏高寒草甸研究6个植物优势种对氮添加的响应,发现该地区的植物生长更易受氮限制。并且我们发现氮添加、磷添加或干旱处理对3种植物生物量的影响不存在交互作用,这说明该区域植物生长的氮限制不受土壤磷有效性和水分的调控。氮磷同时添加、氮和干旱同时处理对2种禾本科植物的生物量呈正效应,但对杂类草不影响,可能是因为高寒草甸氮限制得到缓解后,禾本植物较杂类草冠层更高,不易受到光限制[33-35],导致禾本科植物对包含氮的处理的响应幅度更大[36]。总体上来说,造成氮效应大于磷和水分效应的差异可能有以下几方面原因。第一、氮元素通常被认为是限制高寒生态系统植物生长的主要限制元素[37-39],当有外源氮输入时,会缓解高寒草甸的氮限制作用,有利于植物生长。第二、研究区域年降雨量超过700 mm,且80%都集中在生长季(5—8月),因而水分可能不是限制该生态系统植物生长的主要因子[40-42]。第三、传统研究认为当植物叶片N:P值小于14表示该生态系统植物生长可能受到氮限制,而非磷限制[38,43]。本研究3种植物叶片N:P比值介于7.8 ~ 13.6,表明该高寒草甸植物生长可能不受磷限制。
此外,本研究发现氮添加显著提高3种植物叶片氮含量和N:P比例,磷添加增加植物叶片磷含量同时相应降低N:P比例,这与前人研究结果一致[44-45]。但我们发现干旱处理明显促进禾本科植物叶片氮含量,与传统研究结果相反[46],可能是因为该研究区域的环境特殊性导致的。传统研究通常在干旱和半干旱草原生态系统,干旱会抑制微生物对凋落物和土壤有机氮的分解,进而降低土壤有效氮含量以及植物的吸收和利用[47-48]。而本实验的环境特殊性在于研究区域年降雨量大且集中在生长季,因而水分不是主要限制因子[40-42]。恰恰相反,高寒草甸生长季降雨量大且密集,这会明显促进土壤有效氮(NO3−)的淋溶,相反干旱处理减弱了雨水对土壤有效氮的淋溶作用[49]。同时干旱处理会降低土壤反硝化微生物活性[50-51],减少土壤有效氮因反硝化作用产生的损失。这支持了我们实验得出的干旱促进土壤氮有效性的结果。
同时,我们发现氮添加与干旱处理之间对禾本科植物叶片氮含量和N:P比例有正的交互作用,但是对杂类草草玉梅没有交互影响,可能存在以下几方面原因。第一、氮添加在增加土壤有效氮的同时,干旱通过减少土壤有效氮的淋溶,进而间接地增加土壤有效氮含量,两者共同作用放大了对土壤有效氮的影响效应,最终也有利于植物的氮吸收。第二、禾本科植物根系呈伞形细根分布,而杂类草植物根系以主根为主,两类功能群植物根系形态的特异性可能决定了禾本科植物对土壤有效氮更敏感[52]。第三,高寒草甸杂类草植物一般低于禾本科植物,更容易受到光限制,而光限制作用可能会抑制植物生长对土壤有效氮的响应[33]。此外,尽管氮或磷添加分别提高3种植物的叶片氮或磷含量,但是氮添加和磷添加对它们的叶片养分含量没有交互影响,指示出高寒草甸植物养分吸收主要受氮和磷限制[53],但不受氮磷共同限制[54-56]。
4.2 结 论
本实验充分考虑土壤氮和磷养分与干旱交互作用对高寒草甸植物生长和养分性状的影响。我们发现高寒草甸优势植物叶片养分及其平衡对短期养分添加和干旱比植物生长更敏感。氮添加主要刺激了优势植物生物量,而土壤氮、磷有效性和水分均改变了植物叶片氮和磷含量及N:P比例。同时,氮添加和干旱对植物叶片氮含量存在正的交互作用,而对植物生长不影响。本研究意义在于揭示土壤氮、磷与干旱对高寒草甸植物养分复杂的交互影响,有助于我们理解高寒草甸植物生理过程对全球变化交互作用的响应机理,并为草地生态系统管理提供科学依据。
-
图 1 研究样地位置与小区布置示意图
CK. 对照;+N. 氮添加;+P. 磷添加;D. 干旱(减雨50%);N + P. 氮磷同时添加;N + D. 氮添加和减雨50%。下同。CK, control; +N, nitrogen addition; +P, phosphorus addition; D, drought (50% rainfall reduction); N + P, nitrogen and phosphorus adding simultaneously; N + D, nitrogen addition and drought treatment. The same below.
Figure 1. Sketch map of the location of research sample plots and layout of experimental plots
图 2 3种植物地上生物量对氮添加(+ N)、磷添加(+ P)、干旱(D)、氮磷同时添加(N + P)、氮和干旱同时处理(N + D)的响应
*表示处理与对照差异显著(P < 0.05)。* indicates a significant difference between treatment and control (P < 0.05).
Figure 2. Responses of aboveground biomass in three species to nitrogen addition (+ N), phosphorus addition (+ P), drought (D) , nitrogen and phosphorus adding simultaneously (N + P), and nitrogen addition and drought simultaneously (N + D)
图 3 3种植物叶片氮、磷含量及N:P比例对氮添加(+ N)、磷添加(+ P)、干旱(D)、氮磷同时添加(N + P)、氮和干旱同时处理(N + D)的响应
*表示处理与对照相比差异显著(P < 0.05),黑色柱子表示交互作用显著,虚线表示与交互项比较的2个处理的平均值。* indicates a significant difference between treatment and control (P < 0.05). Black bar indicates a significant interaction effect, and the dashed line indicates the average of the two treatments compared with the interaction item.
Figure 3. Responses of leaf nitrogen, phosphorus concentration and N:P ratio in three plant species to nitrogen addition (+ N), phosphorus addition (+ P), drought (D), nitrogen and phosphorus adding simultaneously (N + P), and nitrogen addition and drought simultaneously (N + D)
表 1 氮添加(+ N)、磷添加(+ P)、干旱(D)及其交互作用(N × P,N × D)对植物生物量、叶片氮含量、磷含量和N:P比例影响的三因素方差分析
Table 1 Three-way ANOVA on the effects of nitrogen addition (+ N), phosphorus addition (+ P), drought (D) and their interactions (N × P, N × D) on plant biomass, leaf nitrogen concentration, phosphorus concentration and N:P ratios
物种 Species 指标 Index df + N + P D N × P N × D 垂穗披碱草
Elymus nutans生物量 Biomass 1 14.501 1** 1.059 4 0.068 4 0.765 8 0.151 0 氮含量 Nitrogen content 1 17.853 8*** 1.166 6 9.793 2** 1.511 7 4.198 7* 磷含量 Phosphorus content 1 3.550 0 93.236 8*** 0.279 5 0.152 9 1.117 3 N:P 1 31.713 9*** 85.326 3*** 3.112 7 3.829 9 9.121 1** 发草
Deschampsia caespitosa生物量 Biomass 1 54.514 0*** 0.550 5 3.326 9 0.307 1 0.410 3 氮含量 Nitrogen content 1 17.213 7*** 0.037 2 12.443 2** 0.990 2 5.689 5* 磷 含量Phosphorus content 1 0.293 0 26.904 9*** 0.273 2 0.107 6 1.845 0 N:P 1 5.572 1* 69.735 5*** 0.001 9 0.245 9 6.550 4* 草玉梅
Anemone rivularis生物量 Biomass 1 10.190 5* 0.688 7 0.238 8 0.074 9 0.060 0 氮含量 Nitrogen content 1 4.584 5* 2.286 1 1.747 6 0.099 6 0.106 8 磷含量 Phosphorus content 1 2.995 6 9.581 3** 0.268 8 0.053 9 3.304 0 N:P 1 15.580 5*** 24.599 6*** 4.304 1 0.140 3 1.695 2 注:*表示在P < 0.05水平上差异显著;**表示在P < 0.01水平上差异显著;***表示在P < 0.001水平上差异显著。Notes: *, **, *** represent significant difference at P < 0.05, P < 0.01 and P < 0.001 level, respectively. -
[1] Stevens C J, Dise N B, Mountford J O, et al. Impact of nitrogen deposition on the species richness of grasslands[J]. Science, 2004, 303: 1876−1879. doi: 10.1126/science.1094678
[2] Clark C M, Tilman D. Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands[J]. Nature, 2008, 451: 712−717. doi: 10.1038/nature06503
[3] Knapp A K, Beier C, Briske D D, et al. Consequences of more extreme precipitation regimes for terrestrial ecosystems[J]. Bioscience, 2008, 58(9): 811−821. doi: 10.1641/B580908
[4] Elser J J, Bracken M E S, Cleland E E, et al. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems[J]. Ecology Letters, 2007, 10(12): 1135−1142. doi: 10.1111/j.1461-0248.2007.01113.x
[5] Sardans J, Rivas-Ubach A, Peñuelas J. The C:N:P stoichiometry of organisms and ecosystems in a changing world: a review and perspectives[J]. Perspectives in Plant Ecology, Evolution and Systematics, 2012, 14(1): 33−47. doi: 10.1016/j.ppees.2011.08.002
[6] Cox P M, Betts R, Jones C D, et al. Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model[J]. Nature, 2000, 408: 184−187. doi: 10.1038/35041539
[7] Wright I J, Westoby M. Nutrient concentration, resorption and lifespan: leaf traits of Australian sclerophyll species[J]. Functional Ecology, 2003, 17(1): 10−19. doi: 10.1046/j.1365-2435.2003.00694.x
[8] Craine J M, Tilman D, Wedin D, et al. Functional traits, productivity and effects on nitrogen cycling of 33 grassland species[J]. Functional Ecology, 2002, 16(5): 563−574.
[9] Cernusak L A, Winter K, Turner B L. Leaf nitrogen to phosphorus ratios of tropical trees: experimental assessment of physiological and environmental controls[J]. New Phytologist, 2010, 185(3): 770−779. doi: 10.1111/j.1469-8137.2009.03106.x
[10] Matzek V, Vitousek P M. N: P stoichiometry and protein: RNA ratios in vascular plants: an evaluation of the growth-rate hypothesis[J]. Ecology Letters, 2009, 12(8): 765−771. doi: 10.1111/j.1461-0248.2009.01310.x
[11] Niklas K J, Owens T, Reich P B, et al. Nitrogen/phosphorus leaf stoichiometry and the scaling of plant growth[J]. Ecology Letters, 2005, 8(6): 636−642. doi: 10.1111/j.1461-0248.2005.00759.x
[12] Baldwin D S, Mitchell A M. The effects of drying and re-flooding on the sediment and soil nutrient dynamics of lowland river-floodplain systems: a synthesis[J]. Regulated Rivers: Research & Management, 2000, 16(5): 457−467.
[13] Heidari M, Karami V. Effects of different mycorrhiza species on grain yield, nutrient uptake and oil content of sunflower under water stress[J]. Journal of the Saudi Society of Agricultural Sciences, 2014, 13(1): 9−13. doi: 10.1016/j.jssas.2012.12.002
[14] Ali Q, Haider M Z, Iftikhar W, et al. Drought tolerance potential of Vigna mungo L. lines as deciphered by modulated growth, antioxidant defense, and nutrient acquisition patterns[J]. Brazilian Journal of Botany, 2016, 39(3): 801−812. doi: 10.1007/s40415-016-0282-y
[15] Koerselman W, Meuleman A F M. The vegetation N: P ratio: a new tool to detect the nature of nutrient limitation[J]. Journal of Applied Ecology, 1996, 33(6): 1441−1450. doi: 10.2307/2404783
[16] Aerts R, Chapin Ⅲ F S. The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns[J]. Advances in Ecological Research, 1999, 30: 1−67.
[17] Stevens M H H, Shirk R, Steiner C E. Water and fertilizer have opposite effects on plant species richness in a mesic early successional habitat[J]. Plant Ecology, 2006, 183(1): 27−34. doi: 10.1007/s11258-005-9003-5
[18] Sterner R W, Elser J J. Ecological stoichiometry : the biology of elements from molecules to the biosphere[M]. Princeton: Princeton University Press, 2002.
[19] Gǜsewell S. N:P ratios in terrestrial plants: variation and functional significance[J]. New Phytologist, 2004, 164: 243−266. doi: 10.1111/j.1469-8137.2004.01192.x
[20] 宾振钧, 王静静, 张文鹏, 等. 氮肥添加对青藏高原高寒草甸6个群落优势种生态化学计量学特征的影响[J]. 植物生态学报, 2014, 38(3):231−237. doi: 10.3724/SP.J.1258.2014.00020 Bin Z J, Wang J J, Zhang W P, et al. Effects of N addition on ecological stoichiometric characteristics in six dominant plant species of alpine meadow on the Qinghai-Xizang Plateau, China[J]. Chinese Journal of Plant Ecology, 2014, 38(3): 231−237. doi: 10.3724/SP.J.1258.2014.00020
[21] 王长庭, 王根绪, 刘伟, 等. 施肥梯度对高寒草甸群落结构、功能和土壤质量的影响[J]. 生态学报, 2013, 33(10):3103−3113. doi: 10.5846/stxb201202200232 Wang C T, Wang G X, Liu W, et al. Effects of fertilization gradients on plant community structure and soil characteristics in alpine meadow[J]. Acta Ecologica Sinica, 2013, 33(10): 3103−3113. doi: 10.5846/stxb201202200232
[22] Dong J, Cui X, Wang S, et al. Changes in biomass and quality of alpine steppe in response to N & P fertilization in the Tibetan Plateau[J/OL]. PLoS One, 2016, 11(5): e0156146 [2019−04−14]. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0156146.
[23] Wang J, Wang Z, Zhang X, et al. Response of Kobresia pygmaea and Stipa purpurea grassland communities in northern Tibet to nitrogen and phosphate addition[J]. Mountain Research and Development, 2015, 35(1): 78−87. doi: 10.1659/MRD-JOURNAL-D-11-00104.1
[24] Yang Y, Gao Y, Wang S, et al. The microbial gene diversity along an elevation gradient of the Tibetan grassland[J]. The ISME Journal, 2014, 8(2): 430. doi: 10.1038/ismej.2013.146
[25] Wang C, Long R, Wang Q, et al. Fertilization and litter effects on the functional group biomass, species diversity of plants, microbial biomass, and enzyme activity of two alpine meadow communities[J]. Plant and Soil, 2010, 331(1−2): 377−389. doi: 10.1007/s11104-009-0259-8
[26] Tang L, Han W, Chen Y, et al. Resorption proficiency and efficiency of leaf nutrients in woody plants in eastern China[J]. Journal of Plant Ecology, 2013, 6(5): 408−417. doi: 10.1093/jpe/rtt013
[27] Lü X T, Han X G. Nutrient resorption responses to water and nitrogen amendment in semi-arid grassland of Inner Mongolia, China[J]. Plant and Soil, 2010, 327(1−2): 481−491. doi: 10.1007/s11104-009-0078-y
[28] Zhao G, Shi P, Wu J, et al. Foliar nutrient resorption patterns of four functional plants along a precipitation gradient on the Tibetan Changtang Plateau[J]. Ecology and Evolution, 2017, 7(18): 7201−7212. doi: 10.1002/ece3.3283
[29] 张方月. 高寒草甸生态系统碳循环对降雨梯度的响应及机理研究[D]. 北京: 中国科学院地理科学与资源研究所, 2018. Zhang F Y. Responses of ecosystem carbon cycle to precipitation gradient in an alpine meadow [D]. Beijing: Institute of Geographic Sciences and Natural Resources Research, 2018.
[30] Borer E T, Harpole W S, Adler P B, et al. Finding generality in ecology: a model for globally distributed experiments[J]. Methods in Ecology and Evolution, 2014, 5(1): 65−73. doi: 10.1111/2041-210X.12125
[31] Smith M, Sala O, Phillips R. Drought-Net: a global network to assess terrestrial ecosystem sensitivity to drought[EB/OL]. [2019−03−14]. http://asm2015.lternet.edu/working-groups/drought-net-global-network-assess-terrestrial-ecosystem-sensitivity-drought.
[32] Güsewell S. Responses of wetland graminoids to the relative supply of nitrogen and phosphorus[J]. Plant Ecology, 2005, 176(1): 35−55. doi: 10.1007/s11258-004-0010-8
[33] Chapin Ⅲ F S, Shaver G R, Giblin A E, et al. Responses of arctic tundra to experimental and observed changes in climate[J]. Ecology, 1995, 76(3): 694−711. doi: 10.2307/1939337
[34] Shaver G R, Bret-Harte M S, Jones M H, et al. Species composition interacts with fertilizer to control long-term change in tundra productivity[J]. Ecology, 2001, 82(11): 3163−3181. doi: 10.1890/0012-9658(2001)082[3163:SCIWFT]2.0.CO;2
[35] Mack M C, Schuur E A G, Bret-Harte M S, et al. Ecosystem carbon storage in arctic tundra reduced by long-term nutrient fertilization[J]. Nature, 2004, 431: 440−443. doi: 10.1038/nature02887
[36] DeMalach N, Zaady E, Kadmon R. Contrasting effects of water and nutrient additions on grassland communities: a global meta-analysis[J]. Global Ecology and Biogeography, 2017, 26(8): 983−992. doi: 10.1111/geb.12603
[37] Niklaus P A, Leadley P W, Stöcklin J, et al. Nutrient relations in calcareous grassland under elevated CO2[J]. Oecologia, 1998, 116(1−2): 67−75. doi: 10.1007/s004420050564
[38] Han W, Luo Y, Du G. Effects of clipping on diversity and above-ground biomass associated with soil fertility on an alpine meadow in the eastern region of the Qinghai-Tibetan Plateau[J]. New Zealand Journal of Agricultural Research, 2007, 50(3): 361−368. doi: 10.1080/00288230709510304
[39] Chaves M M, Maroco J P, Pereira J S. Understanding plant responses to drought:from genes to the whole plant[J]. Functional Plant Biology, 2003, 30(3): 239−264. doi: 10.1071/FP02076
[40] Klein J A, Harte J, Zhao X Q. Experimental warming causes large and rapid species loss, dampened by simulated grazing, on the Tibetan Plateau[J]. Ecology Letters, 2004, 7(12): 1170−1179. doi: 10.1111/j.1461-0248.2004.00677.x
[41] Bedia J, Busqué J. Productivity, grazing utilization, forage quality and primary production controls of species-rich alpine grasslands with N ardus stricta in northern S pain[J]. Grass and Forage Science, 2013, 68(2): 297−312. doi: 10.1111/j.1365-2494.2012.00903.x
[42] Li H, Zhang F, Li Y, et al. Thirty-year variations of above-ground net primary production and precipitation-use efficiency of an alpine meadow in the north-eastern Qinghai-Tibetan Plateau[J]. Grass and Forage Science, 2016, 71(2): 208−218. doi: 10.1111/gfs.12165
[43] He J S, Wang L, Flynn D F B, et al. Leaf nitrogen: phosphorus stoichiometry across Chinese grassland biomes[J]. Oecologia, 2008, 155(2): 301−330.
[44] Henry H A L, Cleland E E, Field C B, et al. Interactive effects of elevated CO2, N deposition and climate change on plant litter quality in a California annual grassland[J]. Oecologia, 2005, 142(3): 465−473. doi: 10.1007/s00442-004-1713-1
[45] Xia J, Wan S. Global response patterns of terrestrial plant species to nitrogen addition[J]. New Phytologist, 2008, 179(2): 428−439. doi: 10.1111/j.1469-8137.2008.02488.x
[46] Li J, Yang C, Liu X, et al. Inconsistent stoichiometry response of grasses and forbs to nitrogen and water additions in an alpine meadow of the Qinghai-Tibet Plateau[J]. Agriculture, Ecosystems & Environment, 2019, 279: 178−186.
[47] Liu P, Huang J, Han X, et al. Differential responses of litter decomposition to increased soil nutrients and water between two contrasting grassland plant species of Inner Mongolia, China[J]. Applied Soil Ecology, 2006, 34(2−3): 266−275. doi: 10.1016/j.apsoil.2005.12.009
[48] Wang C, Wan S, Xing X, et al. Temperature and soil moisture interactively affected soil net N mineralization in temperate grassland in northern China[J]. Soil Biology and Biochemistry, 2006, 38(5): 1101−1110. doi: 10.1016/j.soilbio.2005.09.009
[49] 范丙全, 胡春芳. 灌溉施肥对壤质潮土硝态氮淋溶的影响[J]. 植物营养与肥料学报, 1998, 4(1):16−21. doi: 10.3321/j.issn:1008-505X.1998.01.003 Fan B Q, Hu C F. Effects of irrigation and fertilization on nitrate leaching in loamy fluvo-aquic soil[J]. Plant Nutrition and Fertilizer Science, 1998, 4(1): 16−21. doi: 10.3321/j.issn:1008-505X.1998.01.003
[50] Scholefield D, Tyson K C, Garwood E A, et al. Nitrate leaching from grazed grassland lysimeters: effects of fertilizer input, field drainage, age of sward and patterns of weather[J]. Journal of Soil Science, 1993, 44(4): 601−613. doi: 10.1111/j.1365-2389.1993.tb02325.x
[51] Ding K, Zhong L, Xin X P, et al. Effect of grazing on the abundance of functional genes associated with N cycling in three types of grassland in Inner Mongolia[J]. Journal of Soils and Sediments, 2015, 15(3): 683−693. doi: 10.1007/s11368-014-1016-z
[52] Liu H, Mi Z, Lin L, et al. Shifting plant species composition in response to climate change stabilizes grassland primary production[J]. Proceedings of the National Academy of Sciences, 2018, 115(16): 4051−4056. doi: 10.1073/pnas.1700299114
[53] Luo X, Mazer S J, Guo H, et al. Nitrogen: phosphorous supply ratio and allometry in five alpine plant species[J]. Ecology and Evolution, 2016, 6(24): 8881−8892. doi: 10.1002/ece3.2587
[54] Zhang J, Yan X, Su F, et al. Long-term N and P additions alter the scaling of plant nitrogen to phosphorus in a Tibetan alpine meadow[J]. Science of the Total Environment, 2018, 625: 440−448. doi: 10.1016/j.scitotenv.2017.12.292
[55] Mamolos A P, Vasilikos C V, Veresoglou D S. Vegetation in contrasting soil water sites of upland herbaceous grasslands and N: P ratios as indicators of nutrient limitation[J]. Plant and Soil, 2005, 270(1): 355−369. doi: 10.1007/s11104-004-1793-z
[56] Rejmánková E, Macek P, Epps K. Wetland ecosystem changes after three years of phosphorus addition[J]. Wetlands, 2008, 28(4): 914−927. doi: 10.1672/07-150.1



 下载:
下载: