Hydraulic characteristics and embolism repair of Populus alba × P. glandulosa after drought stress and rehydration
-
摘要:目的 研究植物遭受不同程度干旱胁迫时,其水力学特性的变化及响应,以及复水后植物栓塞修复能力,为植物应对干旱环境的能力提供水力学依据。方法 以84K杨扦插苗为研究对象,进行渐进的控水处理,根据植株形态特征的变化,分别控水至植株叶面积停止生长、整株萎蔫、50%的叶片死亡及全部叶片死亡4个阶段,而后各阶段植株均进行复水至新生叶片长出。分别在控水和复水处理完成后,测定各阶段植株的木质部水势、叶水势、栓塞脆弱性、枝条导水率及栓塞程度(PLC)等水力学特征,同时测定导管直径、导管连接度及导管抗垮塌能力等木质部解剖结构特征。结果 随着干旱胁迫程度加剧,84K杨叶水势及木质部水势均降低,栓塞加剧,栓塞脆弱性减小,木质部导管直径较对照组显著减小,导管连接度及导管抗垮塌能力显著增大。当植株有50%的叶片死亡时,茎的PLC为44%,当叶片全部死亡时,茎的PLC达65%。复水10 ~ 24 d后,各干旱阶段植株木质部水势及叶水势均恢复至对照组水平,茎的导水率均有所增加,栓塞程度均降低,当茎的PLC恢复至28% ~ 37%时,植株顶端重新展开了3片新叶。叶片全部死亡的植株复水至长出新叶时,茎的PLC显著减少,但植株直径并未增大,即复水阶段没有新生导管参与导水过程,表明茎导水率的恢复是由于发生了栓塞修复。结论 干旱胁迫会对植物水力特性造成不利影响,但植物也会通过改变木质部结构特征来适应环境。植物在维持叶片存活与茎导水能力之间存在一定的权衡,干旱胁迫下会牺牲叶片来维持茎的导水率。但当干旱胁迫解除后,植物的水力学特性也能得到恢复,即使整株叶片死亡的植物,复水后仍能恢复生长,叶片死亡并不能作为判断植物死亡的指标。植物恢复生长与茎导水率恢复之间存在很强的相关性,栓塞能否修复是植物经历干旱后能否存活的主要因素。Abstract:Objective The aim of this study was to explore the changes and responses of hydraulic characteristics of plants under different levels of drought stress, and their ability to repair embolism after rehydration, thereby providing theoretical hydraulic evidences for plants to adapt to drought conditions and their ability to recover after drought.Method 84K poplar (Populus alba × P. glandulosa) cuttings were potted and subjected to a progressive drought. According to the changes of plant morphological characteristics, water was controlled to four stages, i.e. the cessation of leaf expansion, whole plant wilting, 50% leaf mortality and 100% leaf mortality. Then, all plants of each stage were rewatered until new leaves appeared. After water control and rehydration treatment, hydraulic characteristics, such as xylem water potential, leaf water potential, vulnerability to xylem cavitation, stem hydraulic conductivity, the percentage loss of xylem conductance, and xylem anatomy such as the vessel diameter, contact fraction and vessel implosion resistance were measured at each stage.Result With the intensification of drought stress, leaf water potential and xylem water potential decreased, and the embolism increased, but xylem vulnerability to cavitation increased. Also, compared with the control group, the vessel diameter decreased significantly, and the contact fraction and vessel implosion resistance increased significantly. At the stage of 50% leaf mortality, the percentage loss of hydraulic conductivity (PLC) of stem was 44%, and reached 65% at the stage of 100% leaf mortality. After rehydration for 10−24 days, the xylem water potential and leaf water potential in each stage returned to the level of control group, and the stem hydraulic conductivity increased with the PLC decreased. When the PLC of stem restored to 28%−37%, three new leaves spread out. After the plants with 100% leaf mortality were rehydrated until the emergence of new leaves, the PLC of stem was significantly reduced, but the plant diameter did not increase, which showed no new conduits participated in the water transportation during the rehydration, indicating that the restoration of stem hydraulic conductivity may result from embolism repair.Conclusion Drought stress can adversely affect the hydraulic characteristics of plants, but plants can also adapt to the environment by changing the structure of their xylem. There is a certain trade-off between the plant’s ability to maintain leaf survival and transport water, and the leaves may be sacrificed to maintain the water conductivity of stem under drought stress. However, when drought stress is released, the hydraulic characteristics of the plants can also be restored. Even if plants without survival leaves can resume growth after rehydration, leaf death cannot be used as an indicator of plant death. There is a strong correlation between the recovery of plant growth and the restoration of stem hydraulic conductivity. Embolism repair may be the main reason for the restoration of stem hydraulic conductivity.
-
干旱引起的水分胁迫会导致植物木质部栓塞,从而阻碍了水分长距离运输并且降低了从根部到树冠的水分运输能力[1-2]。因此,干旱期间树木的存活很大程度上取决于树种能否抵御水分胁迫带来的负面影响,以及能否在土壤水分条件恢复后迅速恢复植物功能。最近的研究表明,水力学特性是理解植物对干旱和恢复响应的关键[3]。植物抵抗栓塞的能力常用栓塞脆弱性来描述[2],栓塞脆弱性通常用导水率损失50%时对应的木质部水势(P50)来表示[4]。对杨树(Populus spp.)进行梯度式土壤含水量减少的盆栽控制实验表明,随着水分胁迫的加剧,木质部水势降低,栓塞程度加剧,茎的导水能力降低,但枝条的栓塞脆弱性也减小,植物抵御栓塞的能力增强[5]。但是植物在自然环境中,所遭受的土壤水分胁迫通常是逐渐加剧的动态过程,植物的形态特征变化或许能表现其真实的受旱情况,如叶面积停止生长、植株枯萎及叶片死亡等典型表征。这些形态特征与其水力学特性之间的联系,却少有研究。
植物恢复导水能力主要有两种机制:(1)栓塞导管重新复水,即栓塞修复[6-7];(2)形成新的木质部导管[8-9]。目前植物到底以哪种机制恢复导水能力还存在很大争议。有研究发现一些草本和小型木本植物可以通过根压途径来进行栓塞修复[10];最新的研究使用无损材料的射线计算机断层扫描技术,在云杉(Picea spp.)及耐干燥的蕨类植物中发现了木质部栓塞修复的证据[11-12]。但是另有一些学者的研究表明一些桉属(Eucalyptus spp.)及栎属(Quercus spp.)植物复水72 h及1个月后仍未发现栓塞修复现象[13-14]。杨属植物中84K杨(Populus alba × P. glandulosa)因其生长快,木材质量好和相对较高的抗旱性,成为西北干旱半干旱地区的主要造林树种[15],其受旱之后水力学特性的变化,复水后能否恢复导水能力及其恢复机制的相关研究鲜有报道。本研究选取84K杨作为实验材料,通过控水实验,探究不同程度干旱胁迫下及复水后84K杨水力学特性及栓塞修复能力,旨在为未来干旱事件更加频发的气候背景下,植物应对干旱环境时水力学特性及恢复能力提供理论依据,为干旱、半干旱地区植树造林、植被恢复和林业生产实践中选择抗旱树种提供理论与实践指导。
1. 材料与方法
1.1 实验材料及处理
实验材料为西北农林科技大学实验苗圃生长良好的84K杨。2018年3月中旬,采同一无性系的84K杨插条进行扦插。插条置于装有基质土壤的6 L花盆中,放入西北农林科技大学科研温室中培养。培养期间温室日间温度20 ~ 25 ℃,夜间温度为17 ~ 19 ℃,空气湿度为20% ~ 50%。
2018 年7月中旬,当插条的基茎达到6 mm时,采用“渐进控水法”对植株进行水分管理,即每天通过称取盆栽质量获得蒸腾量,后续每日的浇水量为蒸腾量的70%。植株在逐渐加剧的水分胁迫下,会有不同形态特征表现。根据表现,将控水过程分为4个阶段,每阶段20株。阶段1:控水胁迫至叶面积停止生长(此时植株下部叶开始萎蔫);阶段2:控水胁迫至植株呈最大萎焉状态(叶片和叶柄都萎蔫,但无叶片死亡);阶段3:控水胁迫至50%的叶片枯萎死亡;阶段4:控水胁迫至100%的叶片枯萎死亡(叶片皱缩、干枯脆化但无自然脱落现象)。到达每个控水阶段表现后,采集测定相关指标数据后立即恢复正常浇水,直到各阶段植株顶端生长并完全展开3片新叶,再采集1次数据。同时,以良好浇水的植株作为对照组(阶段0),每2 d浇水1次,至土壤饱和含水量。
1.2 测定指标及方法
1.2.1 生长指标
叶面积生长测定:选取植株最顶部(最幼龄)的6片叶子(包括控水处理开始时已经展开的2片叶子和处理过程中新展开的4片叶子),每2 d测量每片叶子的长度和宽度,计算叶面积。预实验中确定叶面积计算公式为:
A=0.6761a−0.1594(r2=0.995,n=30) 式中:A为实际叶面积,a为叶长宽乘积。
用选定6片叶子的叶面积之和代表该植株叶面积。从控水处理开始持续监测5盆植株,直至其叶面积的平均值不再增长。
直径生长测定:从开始控水处理至整株叶片完全死亡(即阶段4)及复水处理的整个过程中,选10株植物,在枝条中部做标记,每3 ~ 4 d,用游标卡尺测量标记位置的直径,并取10株的平均值,监测直径生长变化情况。
1.2.2 木质部水势
在达到每个控水阶段及复水完成的当日傍晚,选择6根枝条,每根枝条上选择3片成熟且健康的叶片套上锡纸袋。于次日06:30,将其于叶柄处切下立即带回实验室,用压力室法测定叶片水势(model 1515D; PMS Instruments Co., Corvallis, OR, USA),此时的叶片经过套袋遮光平衡,叶片水势等于木质部水势[16]。枝条的木质部水势取3片叶水势的平均值,每个阶段的木质部水势为6根枝条的平均值。
1.2.3 叶水势
采集测定木质部水势叶片的同时,在上述6根枝条上再选择3片成熟且健康的叶片,叶柄处切下立即装入密封的锡纸袋中,并将枝条于基部截断装入黑色塑料袋一并带回实验室。用压力室法测定叶水势,枝条的叶水势值取3片叶水势的平均值,每个阶段的叶水势为6根枝条的平均值。
1.2.4 栓塞程度
在测定完水势的枝条上取直径6 ~ 7 mm,长度27.4 cm的茎段(在水下进行,以防切取时瞬间形成栓塞),利用低压液流计(low pressure flow meter with multi-channels, LPFM)测定茎段的导水率(Kh)及导水率损失百分数(percentage loss of hydraulic conductivity, PLC),用PLC表示栓塞程度。计算6根枝条导水率及栓塞程度的平均值,作为该阶段下的导水率和栓塞程度。栓塞程度计算公式为:
PLC(%)=100(Kmax−Kh)/Kmax 式中:Kh为冲洗前茎段的初始导水率值,Kmax为茎段在0.25 MPa下用0.01 mol/L KCL冲洗10 min后枝条的最大导水率值。
1.2.5 木质部栓塞脆弱性
本研究中使用的离心机(ChinaTron, H2100R型离心机)配备了改进的Cochard转子(中国长沙湘仪离心仪器有限公司),制造商对离心机进行改进,使其允许安装光源、显微镜、数码相机及定制的软件。该特殊转子最初由Cochard等[17]设计,具体描述见文献[18],改进的湘仪版离心机的使用见文献[19]。
将测定完自然栓塞程度的茎段,迅速放入离心机中,在0.03 MPa的起始压力下离心30 min,从而获得一个稳定的最大导水率(Kmax)。通过控制离心速度形成从低到高的压力梯度,记录不同压力下对应的枝条导水率。建立枝条的栓塞脆弱曲线,并计算P50[17, 20]。每个阶段测定6根枝条,取平均值作为该阶段的P50值。
1.2.6 木质部解剖结构
在测定完水势的枝条中部取直径为6 ~ 7 mm,长度为2 cm的茎段,用碱性品红染料在0.20 kPa压力(染色高度约为20 cm)下染色40 min,再用0.01 mol/L KCL溶液在0.13 MPa压力下将染色茎段冲洗5 min,以冲洗掉多余染料。用切片机在染色茎段的中部切取18 μm厚的薄片并制片。将制好的切片放置在Leica DM4000B荧光显微镜下观察,在10 × 20倍数下拍照。每个枝条横切面测量1 500个导管,所获取照片用Win-CELL 2013软件分析,统计导管直径、导管连接度(即选定区域内相邻导管连接长度占该区域中所有导管的周长之和的比例)和导管抗垮塌能力[18, 21-22]。
1.3 数据处理
用 Microsoft Office Excel 2016 软件进行数据整理分析,采用SPSS 20.0分析软件进行统计分析。采用单因素方差分析(One-way ANOVA)比较不同处理间的差异性,P < 0.05表示差异性显著。
2. 结果与分析
2.1 生长指标
在渐进的控水处理下,1 ~ 12 d植株叶面积(图1)和直径呈线性增长(图2),直径增长与对照组无显著差异。12 d后叶面积和直径增长速度减缓,控水15 d叶面积停止增长(阶段1),21 d后直径停止生长。观察发现,控水处理20 d,植株呈最大萎蔫状态(阶段2),控水24 d,50%的叶片死亡(阶段3),整株叶片死亡(阶段4)发生在控水30 d。复水后,阶段1 ~ 4分别在10、12、22及24 d后顶端恢复生长并展开新叶。控水处理至全部叶片死亡的植株,复水至顶端展开新叶的过程中,植株直径并未增长(图2)。
2.2 水力学特性
2.2.1 木质部水势
植株木质部水势在良好的土壤水分条件下(阶段0)是−0.31 MPa,随着干旱胁迫加剧呈显著降低趋势(图3A)。叶面积停止生长时(阶段1),其木质部水势为−0.60 MPa,较对照组显著降低1倍;植株最大萎蔫时(阶段2)木质部水势为−1.00 MPa,较对照组显著降低2倍;50%叶片死亡的植株(阶段3)木质部水势降至−1.47 MPa,与阶段0相比降低了370%。经过不同时间的复水后,阶段1、2和3的植株木质部水势均恢复至对照组水平,此时植株顶端萌生并展开新叶,表现为恢复生长(阶段4植株控水后叶片全部死亡故无法收集相关水势数据)。
![]() 图 3 84K杨不同控水阶段及复水后木质部水势和叶水势不同小写字母表示控水或复水处理下不同阶段间差异性显著,不同大写字母表示同一阶段复水前后的差异性显著(P < 0.05)。下同。Different lowercase letters indicate significant differences in varied stages under drought stress or rehydration, and different capital letters indicate significant difference before and after rehydration at the same stage (P < 0.05). The same below.Figure 3. Xylem water potential and leaf water potential of 84K poplar at different stages under drought stress and rehydration
图 3 84K杨不同控水阶段及复水后木质部水势和叶水势不同小写字母表示控水或复水处理下不同阶段间差异性显著,不同大写字母表示同一阶段复水前后的差异性显著(P < 0.05)。下同。Different lowercase letters indicate significant differences in varied stages under drought stress or rehydration, and different capital letters indicate significant difference before and after rehydration at the same stage (P < 0.05). The same below.Figure 3. Xylem water potential and leaf water potential of 84K poplar at different stages under drought stress and rehydration2.2.2 叶水势
植株叶水势在良好的土壤水分条件下(阶段0)是−0.61 MPa,随着干旱胁迫加剧与木质部水势变化趋势相似(图3B)。阶段1的植株叶水势较阶段0降低了43.17%至−0.87 MPa;阶段2的植株叶水势降低至−1.32 MPa,与对照组相比降低了118.29%;阶段3的植物叶水势显著降低了1.59倍至−1.56 MPa。各阶段植株复水后,阶段1、2和3的植株叶水势均恢复至对照组水平,此时各阶段植株恢复生长。
2.2.3 栓塞程度及最大导水率
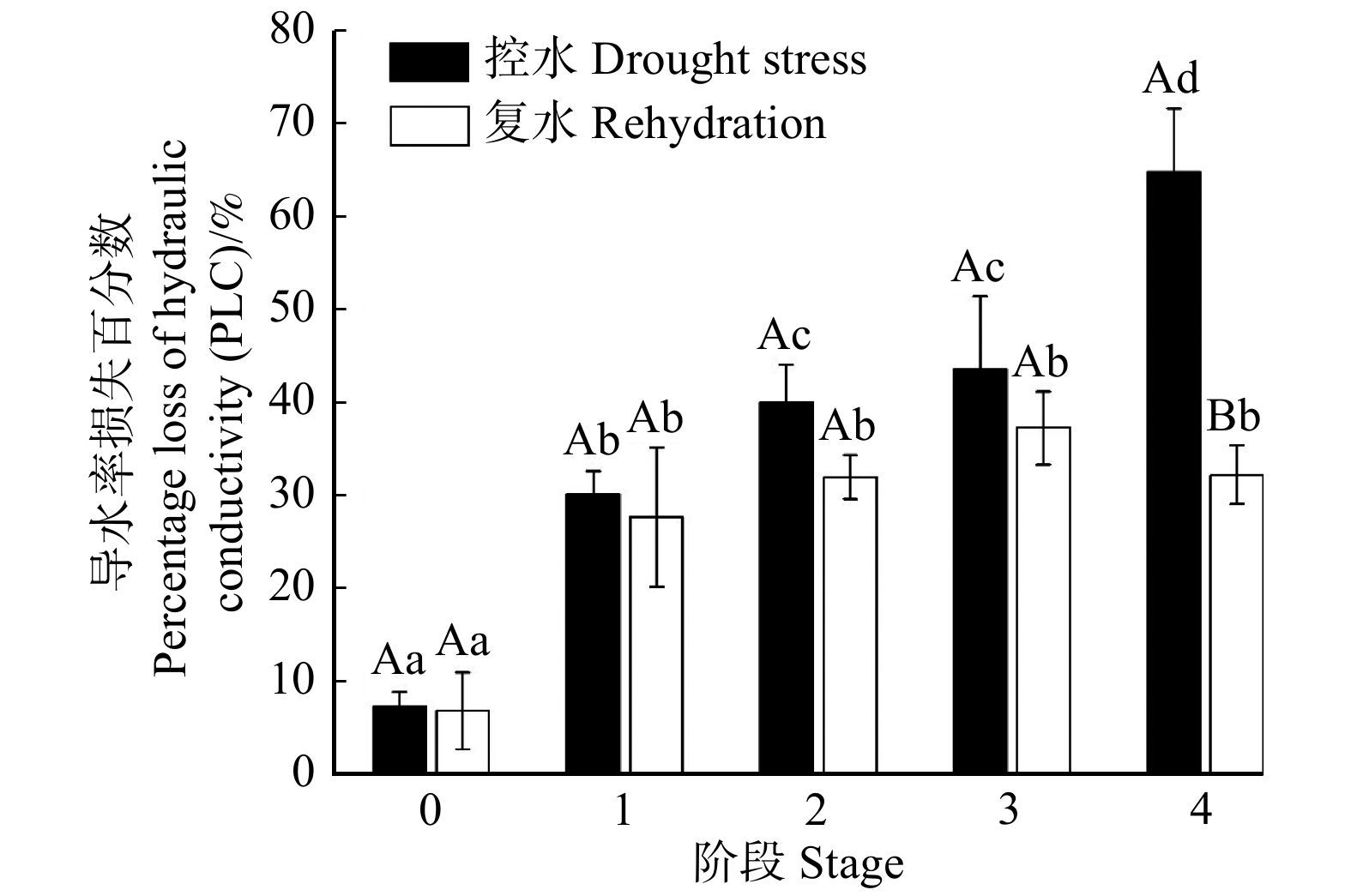
在不同的控水阶段,植株表现出不一样的茎导水率损失程度(PLC),对照组植株茎的PLC为7.46%,随着干旱程度加剧PLC呈逐渐增大趋势(图4)。阶段1的植株茎PLC为30.21%,与对照组相比显著增大了3倍,该阶段的植株表现为叶面积停止生长;阶段2的植株茎PLC显著增大至40.14%,此时植株叶片枯萎;阶段3的植株叶片50%死亡,其茎的PLC为43.63%,但这与阶段2相比未发生显著变化。当叶片全部死亡(阶段4),植株茎的PLC达64.88%,较对照组显著增大了7.7倍。复水后,阶段1、2和3的茎PLC分别降低8.00%、26.65%及14.40%,阶段4复水后的茎PLC降低50.17%,与复水前相比差异性显著。各阶段复水后茎PLC为28% ~ 37%,均未恢复至对照组水平。
土壤水分状况良好的条件下,植株的最大导水率(Kmax)为9.63 × 10−5 kg·m/(MPa·s),随着干旱胁迫不断加剧,植株Kmax呈逐渐降低的趋势(表1)。阶段1的植株Kmax与对照组相比无显著差异,阶段2的Kmax较对照组显著降低54.93%,阶段3显著降低了57.01%,当植株叶片全部死亡时(阶段4),其Kmax较对照组显著降低了71.34%至2.76 × 10−5 kg·m/(MPa·s)。复水后,各阶段植株Kmax与复水前相比均无显著性差异(P > 0.05),且均显著低于对照组水平。
表 1 84K杨不同控水阶段及复水后栓塞脆弱性(P50)及最大导水率Table 1. P50 and maximum hydraulic conductivity of 84K poplar under drought stress and rehydration阶段 Stage 栓塞脆弱性 Embolism vulnerability (P50)/MPa 最大导水率 Maximum hydraulic conductivity (Kmax)/(kg·m·MPa−1·s−1) 控水 Drought stress 复水 Rehydration 控水 Drought stress 复水 Rehydration 0 −1.65 ± 0.048c 9.63 × 10−5 ± 6.60 × 10−6a 1 −1.83 ± 0.022Ab −1.84 ± 0.040Ac 7.70 × 10−5 ± 1.89 × 10−6Aab 5.88 × 10−5 ± 8.02 × 10−6Ab 2 −2.21 ± 0.029Aa −2.09 ± 0.026Ab 4.34 × 10−5 ± 1.66 × 10−6Abc 4.65 × 10−5 ± 8.04 × 10−6Abc 3 −2.23 ± 0.034Aa −2.08 ± 0.087Ab 4.14 × 10−5 ± 9.82 × 10−6Abc 3.60 × 10−5 ± 5.58 × 10−6Ac 4 −2.32 ± 0.034Aa −2.24 ± 0.017Aa 2.76 × 10−5 ± 5.32 × 10−6Ac 3.02 × 10−5 ± 5.28 × 10−6Ac 注:表中数据为平均值 ± 标准误。不同小写字母表示控水或复水下不同阶段间差异性显著,不同大写字母表示同一阶段复水前后的差异性显著(P < 0.05)。下同。Notes: data are mean ± SE. Different lowercase letters indicate significant differences in baried stages under drought stress or rehydration,and different capital letters indicate significant differences before and after rehydration at the same stage (P < 0.05). The same below. 2.2.4 栓塞脆弱性
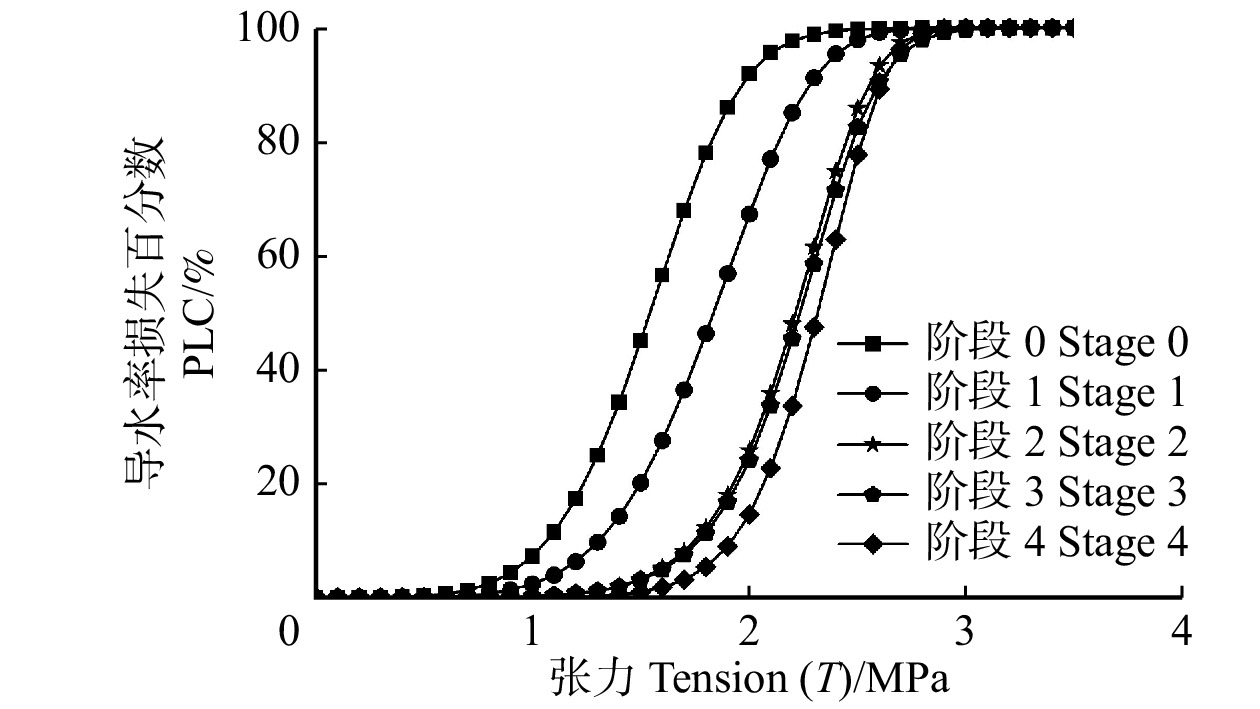
各控水阶段的84K杨枝条栓塞脆弱性曲线均为“S”型(图5),即木质部张力(T)在一定范围内逐渐增大时,枝条导水率(Kh)损失维持在原有水平,只有当T增大到一定程度或水分亏缺达到一定程度时植物才发生栓塞。随着水分胁迫不断加剧,阶段1 ~ 4的栓塞脆弱性曲线与对照组相比,沿着x轴依次向右平移。对照组的枝条栓塞脆弱性(P50)为−1.65 MPa,各阶段枝条的P50表现为阶段1 > 阶段2 > 阶段3 > 阶段4。P50越小,植物栓塞脆弱性越小,抵抗栓塞的能力越强。阶段1的P50与对照组相比减小了10.90%,阶段4较对照组减小了40.60%达到−2.32 MPa,且4个控水阶段的P50值均与对照组差异性显著(表1)。
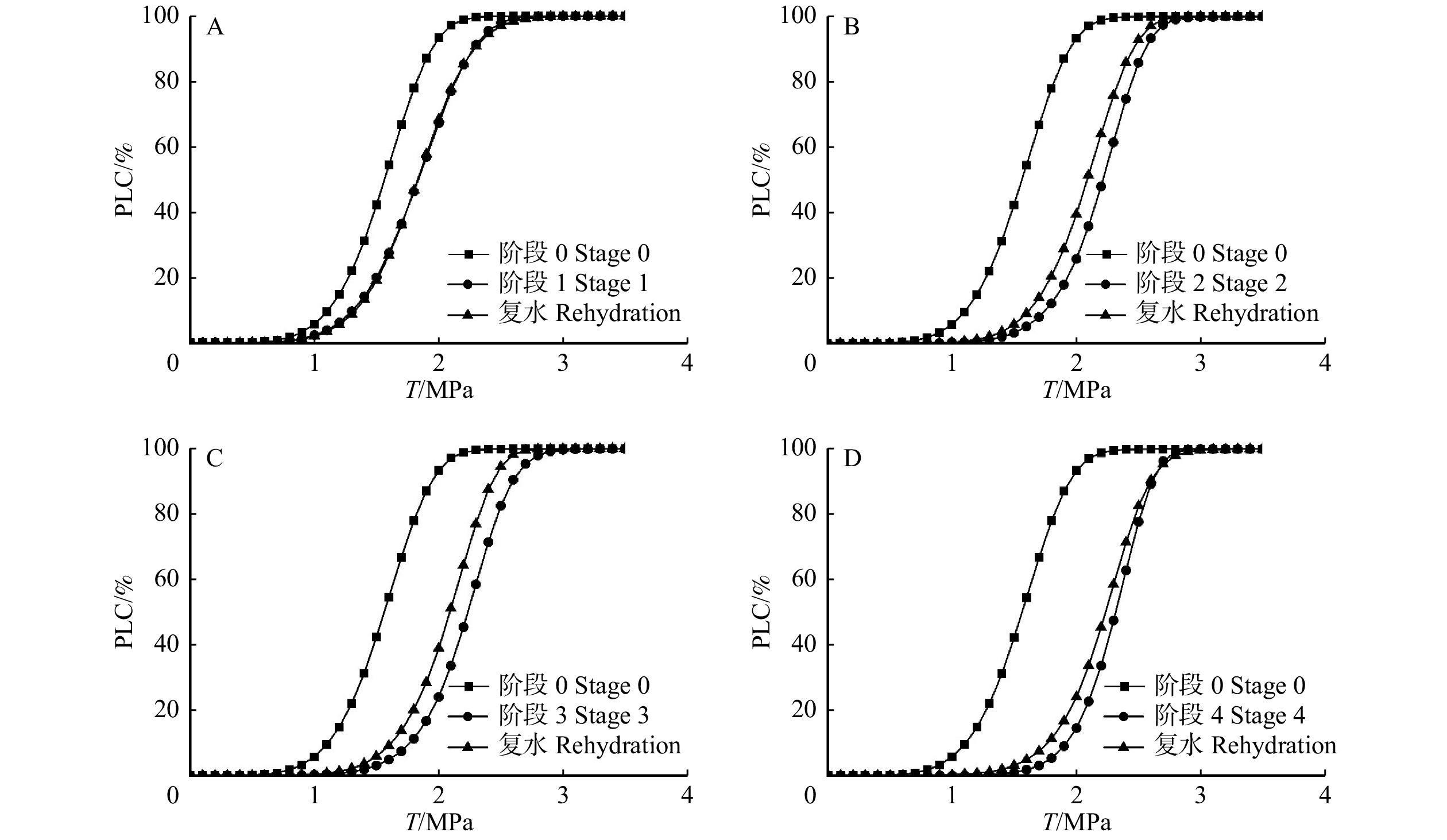
各阶段完成复水后,枝条栓塞脆弱性曲线形状均未发生改变,仍然呈标准的“S”型(图6)。其中,阶段1复水前后的枝条栓塞脆弱性曲线(图6A)和P50(表1)都无显著变化,阶段2、3和4复水后栓塞脆弱性曲线沿x轴轻微左移(图6B、6C、6D),但是其P50(表1)与控水阶段相比无显著性差异(P > 0.05)。植株在经历较为短暂的干旱−复水处理后,其栓塞脆弱性并未显著变化。
2.3 木质部解剖结构特征
在土壤水分条件良好的条件下,植株导管直径为(37.34 ± 0.15) µm,导管连接度为0.03,导管抗垮塌能力为0.04。随着水分胁迫程度加剧,对植株木质部解剖结构产生影响,阶段1、2、3、4与阶段0相比,植株导管直径较对照组显著减小(表2),导管连接度与导管抗垮塌能力较对照组显著增大(P < 0.05),这有利于减小栓塞脆弱性,提高植物在水分胁迫时抵御栓塞的能力[23]。各阶段植株复水后,导管直径、导管连接度及导管抗垮塌能力与复水前无显著性差异(P > 0.05),较短暂的复水处理并未对植株木质部解剖结构产生明显影响。
表 2 84K杨复水前后木质部结构特征Table 2. Xylem structural characteristics of 84K poplar under drought stress and rehydration阶段 Stage 导管直径 Vessel diameter/µm 导管连接度 Vessel contact fraction 导管抗垮塌能力 Vessel implosion resistance 控水 Drought stress 复水 Rehydration 控水 Drought stress 复水 Rehydration 控水 Drought stress 复水 Rehydration 0 37.34 ± 0.15a 0.03 ± 0.001a 0.04 ± 0.003c 1 35.49 ± 0.06Ab 35.63 ± 0.18Ab 0.05 ± 0.003Ab 0.05 ± 0.004Ab 0.06 ± 0.005Aa 0.06 ± 0.003Aa 2 34.97 ± 0.07Ac 34.47 ± 0.16Ac 0.05 ± 0.004Ab 0.05 ± 0.002Ab 0.05 ± 0.001Ab 0.05 ± 0.002Ab 3 34.77 ± 0.20Ac 34.51 ± 0.03Ac 0.05 ± 0.003Ab 0.04 ± 0.005Ab 0.05 ± 0.001Ab 0.05 ± 0.002Ab 4 34.80 ± 0.31Ac 34.85 ± 0.30Ac 0.04 ± 0.005Ab 0.05 ± 0.002Ab 0.05 ± 0.001Ab 0.05 ± 0.003Ab 3. 讨 论
干旱胁迫对植物生长和生存构成了严重的威胁,随着土壤水分条件逐渐恶劣,植物形态特征的变化或能真实地反映其受旱程度,为评估干旱胁迫下植物的水力特性提供了参考指标[24-26]。本研究中,干旱胁迫使得84K杨从良好生长至整株叶片死亡,不同的形态特征反映了植株受旱后水力特性的受损情况:植株叶面积停止生长时,其木质部水势下降50%,枝条导水率损失了1/3;植株叶片死亡反映了其水力特性进一步受损,半数叶片死亡时,其木质部水势下降3.7倍导致其枝条导水率损失近50%。植物叶片这些典型的形态特征变化为了解其真实受旱水平提供了直观的参考依据。
在土壤水分胁迫下,植物的木质部水势降低,植物木质部张力增大,这会导致木质部导管发生栓塞以及造成茎的导水率下降[2]。对照组的木质部水势及茎PLC分别为−0.31 MPa和7%,阶段1和2的木质部水势较对照组均显著降低,其PLC分别增大至30%和40%。阶段3木质部水势进一步显著减小至−1.46 MPa,但是其PLC(43%)却维持在阶段2的水平。这或许是由于阶段2到阶段3的过程中,叶片开始死亡,引起蒸腾面积减小,一定程度上阻止了栓塞进一步增大,从而维持了茎的导水功能,该现象也常见于白杨(Populus tomentosa)中[27],这表明干旱胁迫下植物在维持叶片存活与茎导水能力之间存在一定的权衡。
栓塞脆弱曲线的建立使得人们可以评估植物对栓塞的抵抗力,有些学者分析发现木质部栓塞脆弱性可作为抗旱性的一个指标[26, 28-31]。本研究中,植物在遭受干旱胁迫时,阶段1 ~ 4的P50与对照组相比显著减小(表1),表明水分胁迫下其栓塞脆弱性减小,抵御栓塞的能力增强,一定程度上提高了植物的抗旱性。有学者认为,植物木质部栓塞脆弱性与其最大导水率之间有相关性[5, 21, 32]。本文中,在不断加剧的干旱胁迫下,植株的Kmax较对照组显著减小,这与其P50变化趋势一致,表明干旱胁迫降低了植株的水分运输效率,但有利于减小植株的栓塞脆弱性。在土壤水分亏缺的环境下,植物能通过降低水分运输的效率从而一定程度上提高了水分运输的安全性。前人研究表明,木质部栓塞脆弱性与导管的解剖特征密切相关[30-31, 33-34],阶段1 ~ 4的导管直径较对照组显著减小,导管连接度及导管抗垮塌能力显著增大,这些结构特征的改变都有利于提高木质部对于栓塞的抵抗能力,这与前人研究结果一致[5, 21]。冯枫等学者在离体的84K杨枝条上进行栓塞−复水循环实验后,植物会发生明显的栓塞疲劳现象[35]。但是本实验中,各阶段植株复水后均没有出现显著的栓塞疲劳现象,这或许是由于植物受到的水分胁迫是较为短期且逐渐进行的,所造成的枝条导水率损失程度(最大为65%)低于离心机诱导的栓塞程度(95%以上),且复水后,活体植物所经历的生理活动而非简单的冲洗实验所能替代的,这表明较短期的干旱胁迫后植株并不会发生明显的栓塞疲劳现象。
最近的一些研究表明,为了应对干旱,一些植物能在干旱缓解后,通过栓塞修复来恢复其水力功能[36]。本研究中,复水后当茎PLC在28% ~ 37%,各阶段植株都能恢复生长,表明植株生长新叶与枝条维持一定的导水能力之间存在关系。阶段1植株恢复生长用时最短,仅复水10 d,阶段4用时最长(24 d),这是因为不同阶段的植株在控水期受到的干旱胁迫程度不同,受旱程度越重,恢复时间越久。阶段4植株复水24 d后,其PLC降低了50%,但是植株直径并未显著增大(图2),这排除了新生导管参与导水的影响,从而证明干旱胁迫引起的木质部栓塞是可以修复的,这进一步支持了前人在美洲白杨(Populus trichocarpa)复水后发现的栓塞修复现象[36-39]。
4. 结 论
逐渐加剧的干旱胁迫会使84K杨叶水势、木质部水势及茎导水率降低,枝条栓塞脆弱性减小,抵御栓塞的能力增强。84K杨复水后,不同受旱程度的植物叶水势及木质部水势均恢复至对照组水平。植物复水后恢复生长的能力不同,受旱程度高的植物恢复较慢,但即使整株叶片死亡的植株,也能恢复生长。叶片生长需要茎维持一定的导水率,当茎的PLC恢复至28% ~ 37%,84K杨腋芽就能萌发重新生长新叶。植株茎也许可以通过栓塞修复恢复导水能力,而不依赖于新木质部导管的形成。
-
图 3 84K杨不同控水阶段及复水后木质部水势和叶水势
不同小写字母表示控水或复水处理下不同阶段间差异性显著,不同大写字母表示同一阶段复水前后的差异性显著(P < 0.05)。下同。Different lowercase letters indicate significant differences in varied stages under drought stress or rehydration, and different capital letters indicate significant difference before and after rehydration at the same stage (P < 0.05). The same below.
Figure 3. Xylem water potential and leaf water potential of 84K poplar at different stages under drought stress and rehydration
表 1 84K杨不同控水阶段及复水后栓塞脆弱性(P50)及最大导水率
Table 1 P50 and maximum hydraulic conductivity of 84K poplar under drought stress and rehydration
阶段 Stage 栓塞脆弱性 Embolism vulnerability (P50)/MPa 最大导水率 Maximum hydraulic conductivity (Kmax)/(kg·m·MPa−1·s−1) 控水 Drought stress 复水 Rehydration 控水 Drought stress 复水 Rehydration 0 −1.65 ± 0.048c 9.63 × 10−5 ± 6.60 × 10−6a 1 −1.83 ± 0.022Ab −1.84 ± 0.040Ac 7.70 × 10−5 ± 1.89 × 10−6Aab 5.88 × 10−5 ± 8.02 × 10−6Ab 2 −2.21 ± 0.029Aa −2.09 ± 0.026Ab 4.34 × 10−5 ± 1.66 × 10−6Abc 4.65 × 10−5 ± 8.04 × 10−6Abc 3 −2.23 ± 0.034Aa −2.08 ± 0.087Ab 4.14 × 10−5 ± 9.82 × 10−6Abc 3.60 × 10−5 ± 5.58 × 10−6Ac 4 −2.32 ± 0.034Aa −2.24 ± 0.017Aa 2.76 × 10−5 ± 5.32 × 10−6Ac 3.02 × 10−5 ± 5.28 × 10−6Ac 注:表中数据为平均值 ± 标准误。不同小写字母表示控水或复水下不同阶段间差异性显著,不同大写字母表示同一阶段复水前后的差异性显著(P < 0.05)。下同。Notes: data are mean ± SE. Different lowercase letters indicate significant differences in baried stages under drought stress or rehydration,and different capital letters indicate significant differences before and after rehydration at the same stage (P < 0.05). The same below. 表 2 84K杨复水前后木质部结构特征
Table 2 Xylem structural characteristics of 84K poplar under drought stress and rehydration
阶段 Stage 导管直径 Vessel diameter/µm 导管连接度 Vessel contact fraction 导管抗垮塌能力 Vessel implosion resistance 控水 Drought stress 复水 Rehydration 控水 Drought stress 复水 Rehydration 控水 Drought stress 复水 Rehydration 0 37.34 ± 0.15a 0.03 ± 0.001a 0.04 ± 0.003c 1 35.49 ± 0.06Ab 35.63 ± 0.18Ab 0.05 ± 0.003Ab 0.05 ± 0.004Ab 0.06 ± 0.005Aa 0.06 ± 0.003Aa 2 34.97 ± 0.07Ac 34.47 ± 0.16Ac 0.05 ± 0.004Ab 0.05 ± 0.002Ab 0.05 ± 0.001Ab 0.05 ± 0.002Ab 3 34.77 ± 0.20Ac 34.51 ± 0.03Ac 0.05 ± 0.003Ab 0.04 ± 0.005Ab 0.05 ± 0.001Ab 0.05 ± 0.002Ab 4 34.80 ± 0.31Ac 34.85 ± 0.30Ac 0.04 ± 0.005Ab 0.05 ± 0.002Ab 0.05 ± 0.001Ab 0.05 ± 0.003Ab -
[1] McDowell N, Pockman W T, Allen C D, et al. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought?[J]. New Phytologist, 2008, 178(4): 719−739. doi: 10.1111/j.1469-8137.2008.02436.x
[2] Tyree M T, Sperry J S. Vulnerability of xylem to cavitation and embolism[J]. Annual Review of Plant Biology, 1989, 40(1): 19−36. doi: 10.1146/annurev.pp.40.060189.000315
[3] Creek D, Blackman C J, Brodribb T J, et al. Coordination between leaf, stem, and root hydraulics and gas exchange in three arid-zone angiosperms during severe drought and recovery[J]. Plant, Cell & Environment, 2018, 41(12): 2869−2881.
[4] Domec J C, Gartner B L. Cavitation and water storage in bole segments of mature and young Douglas-fir trees[J]. Trees, 2001, 15(4): 204−214. doi: 10.1007/s004680100095
[5] 李美琦, 姜在民, 赵涵, 等. 加杨水力学与生理特性对不同土壤水分条件响应研究[J]. 植物生理学报, 2017, 53(4):632−640. Li M Q, Jiang Z M, Zhao H, et al. Study on the adaptability of hydraulic and physiological characteristics to different soil moisture conditions in Populus × canadensis Moench[J]. Plant Physiology Journal, 2017, 53(4): 632−640.
[6] Salleo S, Gullo M A L, Paoli D D, et al. Xylem recovery from cavitation-induced embolism in young plants of Laurus nobilis: a possible mechanism[J]. New Phytologist, 1996, 132(1): 47−56. doi: 10.1111/j.1469-8137.1996.tb04507.x
[7] Brodersen C R, McElrone A J, Choat B, et al. The dynamics of embolism repair in xylem: in vivo visualizations using high-resolution computed tomography[J]. Plant Physiology, 2010, 154(3): 1088−1095. doi: 10.1104/pp.110.162396
[8] Améglio T, Bodet C, Lacointe A, et al. Winter embolism, mechanisms of xylem hydraulic conductivity recovery and springtime growth patterns in walnut and peach trees[J]. Tree Physiology, 2002, 22(17): 1211−1220. doi: 10.1093/treephys/22.17.1211
[9] Brodribb T J, Bowman D J M S, Nichols S, et al. Xylem function and growth rate interact to determine recovery rates after exposure to extreme water deficit[J]. New Phytologist, 2010, 188(2): 533−542. doi: 10.1111/j.1469-8137.2010.03393.x
[10] Zwieniecki M A, Holbrook N M. Confronting Maxwell’s demon: biophysics of xylem embolism repair[J]. Trends in Plant Science, 2009, 14(10): 530−534. doi: 10.1016/j.tplants.2009.07.002
[11] Mayr S, Schmid P, Beikircher B, et al. Die hard: timberline conifers survive annual winter embolism[J]. New Phytologist, 2020, 226(1): 13−20. doi: 10.1111/nph.16304
[12] Holmlund H I, Pratt R B, Jacobsen A L, et al. High-resolution computed tomography reveals dynamics of desiccation and rehydration in fern petioles of a desiccation-tolerant fern[J]. New Phytologist, 2019, 224(1): 97−105. doi: 10.1111/nph.16067
[13] Brendan C, Markus N, Rosana L, et al. Non-invasive imaging shows no evidence of embolism repair after drought in tree species of two genera[J]. Tree Physiology, 2018, 39(1): 113−121.
[14] Li X M, Blackman C J, Rymer P D, et al. Xylem embolism measured retrospectively is linked to canopy dieback in natural populations of Eucalyptus piperita following drought[J]. Tree Physiology, 2018, 38(8): 1193−1199. doi: 10.1093/treephys/tpy052
[15] 周永学, 符毓秦, 樊军锋, 等. 84K杨的生长特性及杂交可配性[J]. 东北林业大学学报, 2007, 35(10):13−14. doi: 10.3969/j.issn.1000-5382.2007.10.005 Zhou Y X, Fu Y Q, Fan J F, et al. Growth characteristics and crossability of poplar 84K[J]. Journal of Northeast Forestry University, 2007, 35(10): 13−14. doi: 10.3969/j.issn.1000-5382.2007.10.005
[16] Wang R Q, Zhang L L, Zhang S X, et al. Water relations of Robinia pseudoacacia L. : do vessels cavitate and refill diurnally or are R-shaped curves invalid in Robinia?[J]. Plant, Cell & Environment, 2014, 37(12): 2667−2678.
[17] Cochard H, Damour G, Bodet C, et al. Evaluation of a new centrifuge technique for rapid generation of xylem vulnerability curves[J]. Physiologia Plantarum, 2010, 124(4): 410−418.
[18] Cai J, Tyree M T. The impact of vessel size on vulnerability curves: data and models for within-species variability in saplings of aspen, Populus tremuloides Michx[J]. Plant, Cell & Environment, 2010, 33(7): 1059−1069.
[19] Wang Y, Burlett R, Feng F, et al. Improved precision of hydraulic conductance measurements using a Cochard rotor in two different centrifuges[J/OL]. The Journal of Plant Hydraulics, 2014, 1: e0007 (2014−10−24) [2019−05−05]. https://hal.archives-ouvertes.fr/hal-01077482.
[20] Cochard H. A technique for measuring xylem hydraulic conductance under high negative pressures[J]. Plant, Cell & Environment, 2002, 25(6): 815−819.
[21] 李荣, 姜在民, 张硕新, 等. 木本植物木质部栓塞脆弱性研究新进展[J]. 植物生态学报, 2016, 39(8):838−848. Li R, Jiang Z M, Zhang S X, et al. A review of new research progress on the vulnerability of xylem embolism of woody plants[J]. Chinese Journal of Plant Ecology, 2016, 39(8): 838−848.
[22] 党维, 姜在民, 李荣, 等. 6个树种1年生枝木质部的水力特征及与栓塞修复能力的关系[J]. 林业科学, 2017, 53(3):49−59. doi: 10.11707/j.1001-7488.20170306 Dang W, Jiang Z M, Li R, et al. Relationship between hydraulic traits and refilling of embolism in the xylem of one-year-old twigs of six tree species[J]. Scientia Silvae Sinicae, 2017, 53(3): 49−59. doi: 10.11707/j.1001-7488.20170306
[23] Sperry J S, Hacke U G, Pittermann J. Size and function in conifer tracheids and angiosperm vessels[J]. American Journal of Botany, 2006, 93(10): 1490−1500. doi: 10.3732/ajb.93.10.1490
[24] Engelbrecht B M J, Tyree M T, Kursar T A. Visual assessment of wilting as a measure of leaf water potential and seedling drought survival[J]. Journal of Tropical Ecology, 2007, 23(4): 497−500. doi: 10.1017/S026646740700421X
[25] Tyree M T, Vargas G, Engelbrecht B M J, et al. Drought until death do us part: a case study of the desiccation-tolerance of a tropical moist forest seedling-tree, Licania platypus (Hemsl.) Fritsch[J]. Journal of Experimental Botany, 2002, 53: 2239−2247. doi: 10.1093/jxb/erf078
[26] Tyree M T, Engelbrecht B M J, Vargas G, et al. Desiccation tolerance of five tropical seedlings in Panama relationship to a field assessment of drought performance[J]. Plant Physiology, 2003, 132(3): 1439−1447. doi: 10.1104/pp.102.018937
[27] Braatne J H, Hinckley T M, Stettler R F. Influence of soil water on the physiological and morphological components of plant water balance in Populus trichocarpa, Populus deltoides and their F1 hybrids[J]. Tree Physiology, 1992, 11(4): 325−339. doi: 10.1093/treephys/11.4.325
[28] Sperry J S, Tyree M T. Water-stress-induced xylem embolism in three species of conifers[J]. Plant, Cell & Environment, 1990, 13(5): 427−436.
[29] Cochard H, Breda N, Granier A, et al. Vulnerability to air embolism of three European oak species (Quercus petraea (Matt) Liebl, Q. pubescens Willd, Q. robur L.)[J]. Annals of Forest Science, 1992, 49(3): 225−233. doi: 10.1051/forest:19920302
[30] Cochard H, Barigah S T, Kleinhentz M, et al. Is xylem cavitation resistance a relevant criterion for screening drought resistance among Prunus species?[J]. Journal of Plant Physiology, 2008, 165(9): 976−982. doi: 10.1016/j.jplph.2007.07.020
[31] Maherali H, Pockman W T, Jackson R B. Adaptive variation in the vulnerability of woody plants to xylem cavitation[J]. Ecology, 2004, 85(8): 2184−2199. doi: 10.1890/02-0538
[32] 谢东锋, 马履一, 王华田. 7种造林树种木质部栓塞脆弱性研究[J]. 浙江林学院学报, 2004, 21(2):138−143. Xie D F, Ma L Y, Wang H T. Study on vulnerability of xylem embolism in twigs of seven tree species[J]. Journal of Zhejiang Forestry College, 2004, 21(2): 138−143.
[33] Wheeler J K, Sperry J S, Hacke U G, et al. Inter-vessel pitting and cavitation in woody Rosaceae and other vesselled plants: a basis for a safety versus efficiency trade-off in xylem transport[J]. Plant, Cell & Environment, 2005, 28(6): 800−812.
[34] Hacke U G, Jansen S. Embolism resistance of three boreal conifer species varies with pit structure[J]. New Phytologist, 2009, 182(3): 675−686. doi: 10.1111/j.1469-8137.2009.02783.x
[35] Feng F, Ding F, Tyree M T. Investigations concerning cavitation and frost fatigue in clonal 84K poplar using high-resolution cavitron measurements[J]. Plant Physiology, 2015, 168(1): 144−155. doi: 10.1104/pp.114.256271
[36] Love D M, Sperry J S. In situ embolism induction reveals vessel refilling in a natural aspen stand[J]. Tree Physiology, 2018, 38(7): 1006−1015. doi: 10.1093/treephys/tpy007
[37] Sakr S, Alves G, Morillon R, et al. Plasma membrane aquaporins are involved in winter embolism recovery in walnut tree[J]. Plant Physiology, 2003, 133(2): 630−641. doi: 10.1104/pp.103.027797
[38] Secchi F, Zwieniecki M A. Patterns of PIP gene expression in Populus trichocarpa during recovery from xylem embolism suggest a major role for the PIP1 aquaporin subfamily as moderators of refilling process[J]. Plant, Cell & Environment, 2010, 33(8): 1285−1297.
[39] Secchi F, Gilbert M E, Zwieniecki M A. Transcriptome response to embolism formation in stems of Populus trichocarpa provides insight into signaling and biology of refilling[J]. Plant Physiology, 2011, 157(3): 1419−1429. doi: 10.1104/pp.111.185124
-
期刊类型引用(9)
1. 宫联沙,杨静,戴全厚,聂云鹏,周畅. 不同降雨类型下植被剔除对灌木林降雨再分配特征的影响. 水土保持学报. 2024(05): 315-322+331 .  百度学术
百度学术
2. 任斯宇,张金威,盛后财,琚存勇. 城市蒙古栎人工林对降雨中金属元素迁移的影响. 东北林业大学学报. 2023(02): 33-39 .  百度学术
百度学术
3. 孙天妙,阳辉,曹建生. 太行山区不同植被降雨再分配特征. 中国生态农业学报(中英文). 2023(09): 1471-1481 .  百度学术
百度学术
4. 李连强,杨会侠,丁国泉,李虹谕,白荣芬,王品. 辽宁仙人洞国家级自然保护区森林生态服务物质量评估及权衡与协同. 北京林业大学学报. 2023(09): 83-94 .  本站查看
本站查看
5. 于德水,卢杰. 地被物对土壤含水量及养分影响的研究进展. 吉林林业科技. 2022(01): 24-28 .  百度学术
百度学术
6. 汪水前. 南方水土流失区马尾松对降雨再分配的影响. 中国水土保持科学(中英文). 2022(02): 99-105 .  百度学术
百度学术
7. 王淑春,程然然,杜盛. 黄土丘陵区2种典型林分降雨分配特征及其主要影响因素. 水土保持学报. 2022(03): 173-180 .  百度学术
百度学术
8. 张娜,程琳,赵西平. 蒙古栎生长与材积的研究进展. 防护林科技. 2022(04): 73-74+82 .  百度学术
百度学术
9. 王玉涛,郭雨竹,李佳宁,王澍涵,冀宗琪,陆秀君. 蒙古栎花芽分化、花器官发育及花粉离体萌发特性研究. 沈阳农业大学学报. 2021(05): 530-536 .  百度学术
百度学术
其他类型引用(7)



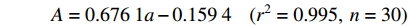


 下载:
下载: