Effects of temperature stress on the accumulation of secondary metabolites and defensive enzymes in multiple shoots of Betula platyphylla
-
摘要:目的 探究温度胁迫对白桦丛生苗三萜、黄酮及抗逆酶积累的影响,为提高白桦次生代谢产物积累以及工厂化生产奠定基础。方法 利用不同浓度(0.5、1.0、2.0和5.0 mg/L)的6-苄氨基腺嘌呤(6-BA),诱导产生白桦丛生苗及建立悬浮培养体系,同时对丛生苗进行温度胁迫诱导次生产物合成。结果 附加2.0 mg/L 6-BA的NT固体培养基可诱导白桦愈伤组织获得白桦丛生苗。2种温度(45和4 ℃)胁迫下,白桦丛生苗中总三萜含量均比对照(25 ℃)有所提高,其中45 ℃处理4 h,恢复培养6 h后,总三萜含量比对照提高5.03倍。4 ℃处理白桦丛生苗1 h,并在24 h时取样,齐墩果酸含量比对照提高了14.52倍,最高含量达2.33 mg/g。4 ℃处理白桦丛生苗4 h,在恢复培养48 h时,黄酮含量比对照提高38.37%。同时明确温度胁迫下,4 ℃胁迫4 h,恢复培养72 h,超氧化物歧化酶(SOD)活性最高,比对照提高69.77%。经4 ℃处理1 h的丛生苗在96 h 时,过氧化氢酶(CAT)活性达到最高峰,是对照的1.81倍。4 ℃处理1 h的丛生苗在恢复培养6 h时抗坏血酸过氧化物酶(APX)活性比对照提高55.29%。当胁迫时间延长至4 h,在45 ℃处理下,黄酮含量与APX酶,SOD与齐墩果酸含量均呈显著负相关(P < 0.05);经4 ℃处理,总三萜与APX(P < 0.01)、CAT(P < 0.05)分别呈极显著正相关和显著正相关;黄酮与SOD含量呈显著负相关(P < 0.05);CAT与APX(P < 0.01)、SOD(P < 0.05)相关性达极显著和显著水平,3种抗逆酶协调发挥作用共同参与次生产物合成。结论 2.0 mg/L的6-BA能成功诱导白桦愈伤组织产生丛生苗,并建立悬浮培养系。短时间高温或低温胁迫均可以刺激3种防御酶发生显著变化,3种酶相互协调共同参与并促进白桦丛生苗总三萜和黄酮物质的合成与积累。Abstract:Objective The effects of temperature stress on the accumulation of triterpenoids, flavonoids and defense enzymes in multiple shoots of birch (Betula platyphylla) were investigated so as to lay the foundation for increasing the accumulation and factory production of secondary metabolites in birch.Method Different concentrations (0.5, 1.0, 2.0 and 5.0 mg/L) of 6-benzylaminoadenine (6-BA) were used to induce multiple shoots and establish suspension culture system of birch. Furthermore, multiple shoots were treated by temperature stress so that the synthesis of secondary metabolites in birch was induced.Result The callus of birch could be induced to obtain the multiple shoots by NT medium supplemented with 2.0 mg/L 6-BA. The content of total triterpenoids in birch multiple shoots under temperature stress (45 and 4 ℃) was higher than that of the control (25 ℃), among them the total triterpenoids content increased by 5.03 times after 4 h and then restoration culture for 6 h. Also the content of oleanolic acid in birch multiple shoots treated by 4 ℃ for 1 h and sampled 24 h later was increased by 14.52 times, and even for 2.33 mg/g. And the content of flavonoids was enhanced by 38.37% under 4 ℃ for 4 h and then 25 ℃ for 48 h. Meanwhile, the superoxide dismutase (SOD) activity was highest under 4 ℃ for 4 h and normal culture for 72 h, which was 69.77% higher than that of control, and the catalase (CAT) activity of birch multiple shoots treated by 4 ℃ for 1 h and normal culture for 96 h reached the peak, which was increased to 1.81 times. Then the ascorbate peroxidase (APX) activity of multiple shoots treated at recovery culture for 6 h under 4 ℃ for 1 h was 55.29% higher than that of control. While treated by 45 ℃ for 4 h, the content of flavonoids and oleanolic acid was significantly negatively correlated (P < 0.05) with APX enzyme and SOD activity, respectively. Furthermore, the content of total triterpenes was extremely significantly positively correlated or significantly positively correlated with APX activity (P < 0.01) and CAT activity (P < 0.05), and the content of flavonoids was significantly negatively correlated (P < 0.05) with SOD activity, also CAT activity was significantly correlated with APX activity (P < 0.01) and SOD activity (P < 0.05). These results showed that the three anti-stress enzymes played their collaboration role in the synthesis of secondary products.Conclusion Multiple shoots of birch were successfully induced by 2.0 mg/L 6-BA, and suspension culture system was established. Short time temperature stress could stimulate the significant changes of three defense enzymes (SOD, CAT and APX), which coordinated with each other and promoted the synthesis and accumulation of total triterpenoids and flavonoids in multiple shoots of birch.
-
Keywords:
- Betula platyphylla /
- multiple shoots /
- suspension culture /
- 6-BA /
- secondary product /
- defensive enzyme
-
白桦(Betula platyphylla)为桦木科(Betulaceae)桦木属植物,是我国东北地区常见树种,富含多种次生代谢物,具有很高的经济和药用价值[1]。如以白桦脂酸、齐墩果酸等为代表的三萜类物质具有抗病毒、抗肿瘤、抗氧化等多种药用活性[2-3]。类黄酮作为抗癌制剂对治疗肺癌、乳腺癌、成胶质细胞瘤等多种恶性肿瘤有显著作用[4]。桦树汁营养丰富、药食兼用,在保健、食品、医药、日化行业均具有价值[5]。近年来,白桦资源被广泛研究和利用,白桦的需求量也日益加大。
传统的树木种植和栽培方法,如种子发芽、扦插、嫁接等具有耗时长,发芽率低、高质量材料短缺等弊端[6]。细胞培养虽耗时短,但也常出现褐化,细胞生长及次生代谢产物合成不稳定等缺点。而丛生苗具有较高的成苗率、次生代谢物产量稳定等优点,更适合于试验研究和规模化发酵培养。王思瑶等[7]以柽柳(Tamarix chinensis)丛生芽为材料,探究培养基与总三萜、总黄酮、总酚产量之间的关系。杨帆等[8]以金线莲(Anoectochilus roxburghii)丛生芽为材料生产较多的多糖、黄酮和酚类物质。李球红等[9]利用桂花(Osmanthus fragrans)丛生苗培养桂花,为桂花的快速繁殖和新品种的培育提供了新的途径和方法。目前,关于白桦丛生苗诱导及其次生代谢产物合成与生产报道较少。
温度(高温和低温)逆境下,具有毒性和高反应性的活性氧(reactive oxygen species,ROS)被过度产生,引起植物发生防御应答反应,最终导致细胞出现过度氧化应激并死亡;而植物自身可在胁迫条件下积累具有抗病、抗虫、抗环境胁迫等生态学功能的次生代谢产物以提高抗逆性[10-12]。这可能是由于H2O2在信号传递过程中激活了由抗坏血酸过氧化物酶(ascorbate peroxidase,APX)、超氧化物歧化酶(superoxide dismutase,SOD)、过氧化氢酶(catalase,CAT)等防御酶组成的抗氧化酶保护系统[13-14]。当植物处于胁迫环境时,防御酶系会做出一系列反应[15]。防御酶活性与活性氧的清洁能力及抵抗胁迫的能力成正比。防御酶活性的变化也导致次级代谢产物的积累。Jumrani等[16]研究发现,温度从22 ℃升高到28 ℃,SOD、APX、POD的活性显著增加。常志凯等[17]通过研究指出高温胁迫诱导白桦悬浮细胞总三萜物质的合成与积累。有研究人员利用低温诱导的方法提高了拟南芥(Arabidopsis thaliana)类黄酮积累量和人参(Panax ginseng)须根总皂苷的积累量[18-19]。乌凤章等[20]通过研究发现,低温(4 ℃)导致白桦高生长基本停止,叶绿素含量逐渐降低。高温(45 ℃)处理可使白桦幼苗部分生长点、叶片边缘变褐[21]。可见,高温和低温胁迫均会对白桦生长和生理产生不利影响,但它对白桦丛生苗次生产物合成及抗逆酶积累有何影响尚不明确。
因此,本研究通过比较不同浓度6-BA作用下白桦丛生苗的生长状况,探究白桦丛生苗最佳培养条件。同时通过4种处理探究温度对白桦三萜、黄酮含量及防御酶活性的影响,以期为提高白桦次生代谢产物以及工厂化生产奠定基础。
1. 材料与方法
1.1 植物材料与培养
试验材料来源于东北林业大学森林生物工程学科培养的白桦愈伤组织。设4种处理,将生长状态良好的愈伤组织转移至附加0.5(T1)、1.0(T2)、2.0(T3)和5.0(T4) mg/L 6-苄氨基腺嘌呤(6-benzylaminopurine,6-BA)的NT固体基本培养基中诱导丛生苗。液体培养基为不加琼脂粉的T1培养基。培养条件为温度25 ℃,光照强度2 000 lx,光照时间16 h/d,湿度40% ~ 50%,摇床转速116 r/min。
白桦愈伤组织在不同激素浓度的NT培养基中被诱导,以4周为1个培养周期,经过两代的转接,建立稳定的丛生苗生长体系。接种5.0 g丛生苗在100 mL液体培养基中悬浮培养,以15 d为1个继代周期,转接6代以上,建立稳定的悬浮培养丛生苗体系。
1.2 不同温度胁迫处理
设置45 ℃ 1 h、45 ℃ 4 h、4 ℃ 1 h、4 ℃ 4 h和对照处理(25 ℃)5个水平处理,丛生苗悬浮培养第7天进行温度胁迫处理,分别在温度胁迫处理后0、6、12、24、48、72和96 h取样收获白桦丛生苗,每个处理重复3次。收获的丛生苗测定鲜质量后,一部分放在−80 ℃冰箱中进行保存,用于抗逆酶活性分析,一部分于烘箱中65 ℃进行烘干,用于测定齐墩果酸含量。
1.3 白桦丛生苗总三萜的提取及含量测定
总三萜的提取及含量测定参考王博等[22]的方法。取2 mL 95%乙醇加入0.05 g丛生苗干样浸泡24 h,70 ℃水浴1 h,超声40 min。吸取上清液100 µL,水浴蒸干。加200 µL 5 %香草醛-冰乙酸溶液、800 µL HCLO4,70 ℃水浴15 min后置于冰上迅速冷却。加乙酸乙酯定容至5 mL。利用分光光度计测定其在551 nm处的吸光值。
1.4 齐墩果酸的提取及含量测定
齐墩果酸的提取方法参照谭朝阳等[23]的方法。丛生苗干样研磨成粉,称取0.50 g,加入25 mL盐酸−乙醇溶液(2∶8),加热回流3 h,放冷,摇匀并过滤,取滤液15 mL,加蒸馏水15 mL,80 ℃水浴蒸去乙醇,然后用乙醚萃取3次,每次20 mL,合并乙醚萃取液并于40 ℃低温蒸干,加1 mL甲醇溶解残渣,利用高效液相色谱检测210 nm处的吸光峰,并计算含量。
1.5 黄酮的提取及含量测定
丛生苗干样研磨成粉,称取0.20 g,加入20 mL 50%乙醇,超声90 min,过滤并定容至25 mL。取4 mL,置于250 mL容量瓶中,加5%亚硝酸钠溶液1 mL,摇匀静置6 min,再加入10%硝酸铝溶液1 mL,摇匀静置6 min,加10%氢氧化钠10 mL,并用50%乙醇定容至刻度,摇匀静置15 min。利用分光光度仪测定551 nm的吸光值,并计算含量。
1.6 防御酶活性测定
酶液A:称取0.20 g丛生苗鲜样置于研钵后,加4.0 mL提酶缓冲液在冰上进行研磨,研磨充分后12 000 r/min离心10 min。酶液B:取0.30 g植物组织鲜样,加入3 mL提酶液,冰浴中充分研磨后,离心。使用酶液A,分别根据施特尔马赫·B[24],李合生[25]的方法测定CAT、SOD的活性。参照赵微[26]方法,使用酶液B测定APX的活性。
1.7 统计分析与关联分析
所得数据均使用office 2010 Excel软件及DPS统计软件进行数据处理和分析。
2. 结果与分析
2.1 丛生苗诱导
将白桦愈伤组织分别转接至T2、T3、T4培养基中诱导丛生苗(图1)。通过观察,诱导培养基T3诱导丛生苗的效率最高,不仅生长速度快、长势好,不定芽诱导率、每块平均不定芽数显著优于其他诱导培养基(表1)。
表 1 白桦愈伤组织不定芽分化Table 1. Differentiation of adventitiousbuds from birch callus处理
Treatment6-BA质量浓度
6-BA mass
concentration/
(mg·L−1)不定芽诱导率
Adventitious
bud induction
rate/%每块平均不定芽数
Average number
of adventitious
buds per pieceT2 1.0 46.67 3 T3 2.0 63.33 6 T4 5.0 53.33 4 2.2 白桦丛生苗悬浮培养体系生长曲线
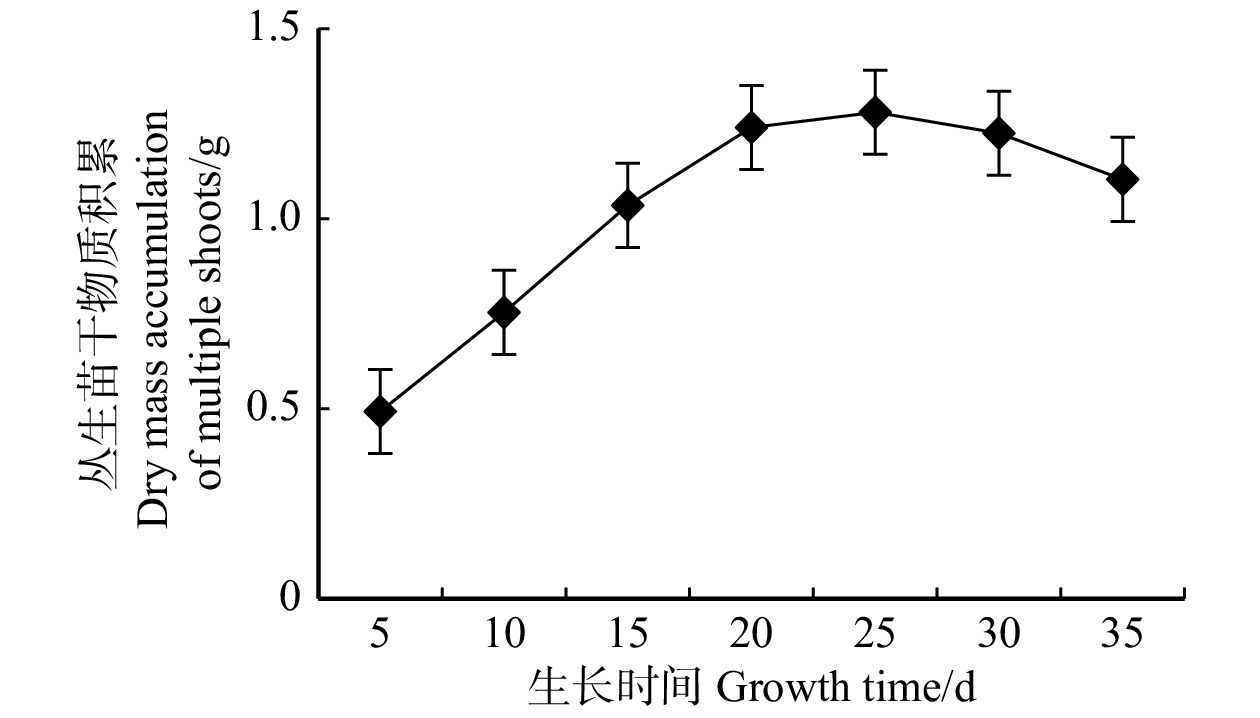
将白桦愈伤组织接种于诱导培养基T3中诱导,产生白桦丛生苗,继代两次后转移至固体培养基T1中扩繁,将上述丛生苗接种于液体培养基中,培养35 d,每隔5 d取样1次。在5 ~ 25 d其干物质的积累呈线性增加,且第25天达到峰值,5 ~ 15 d增长速率最快,25 ~ 35 d因培养液中营养物质消耗殆尽,白桦丛生苗干物质积累也逐渐降低(图2)。
2.3 白桦丛生苗的三萜、齐墩果酸含量的积累
白桦丛生苗悬浮培养至第10天总三萜含量达到最高76.41 mg/g,其次继代30 d时总三萜含量到达第2个峰值73.95 mg/g。5 ~ 10 d积累速率最大,从整体上看总三萜积累比较稳定,且积累量较高(图3A)。
齐墩果酸含量的积累在悬浮培养5 ~ 15 d、25 ~ 30 d呈上升趋势,5 ~ 15 d积累速率最大,且第15天齐墩果酸的积累量最高,15 ~ 25 d齐墩果酸含量逐渐下降,分析原因可能是5 ~ 15 d碳源充足有利于次生产物合成,培养15 d后因碳源消耗而有所降低,20 d后因营养缺乏导致胁迫造成了初生生长降低而刺激次生产物再次合成(图3B)。
2.4 温度胁迫对白桦丛生苗三萜和黄酮物质积累的调节效应
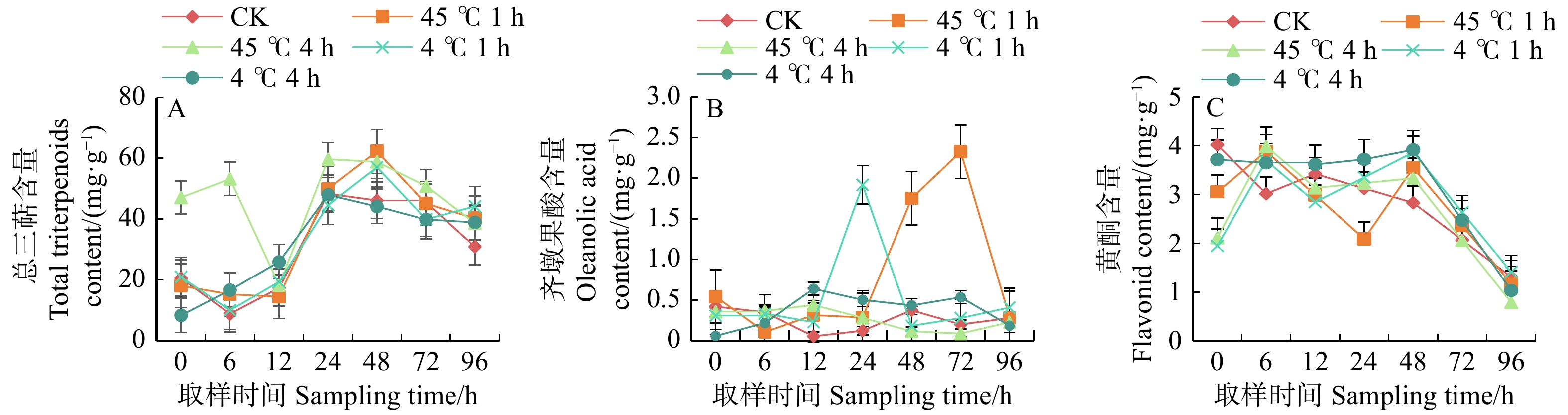
为进一步刺激次生代谢产物合成,对上述的白桦丛生苗在悬浮培养至第7天施加4种不同温度胁迫处理,同时测定胁迫后细胞的抗逆酶SOD、CAT和APX活性的变化。结果表明,热刺激比冷胁迫对于总三萜含量的提高更有利。45 ℃处理白桦丛生苗4 h后恢复培养6 h取样,发现总三萜含量增幅最大,比对照(CK)提高5.03倍,在恢复培养24 h时达到峰值,且相比CK提高了23.42%。4种处理下总三萜含量在恢复培养96 h,均高于CK。45 ℃处理1 h的白桦组培苗在恢复培养48 h总三萜含量达到最高,比CK提高34.89%(图4A)。
温度胁迫后12 ~ 24 h齐墩果酸含量显著均高于CK。4 ℃处理白桦丛生苗1 h后,在恢复培养24 h取样齐墩果酸含量比CK提高14.52倍。齐墩果酸含量最高的是45 ℃热处理白桦丛生苗1 h,在恢复培养72 h时,其含量为2.33 mg/g,比CK提高10.56倍。4 ℃胁迫1 h较4 h处理测得的齐墩果酸含量增加显著(图4B)。上述分析显示,两种温度胁迫处理总三萜含量比对照提高0.017 4 ~ 5.03倍,其含量最高可达62.25 mg/g;齐墩果酸含量较对照提高0.007 5 ~ 14.52倍,最高含量达2.33 mg/g。
温度胁迫处理白桦组培苗在恢复培养6 h和48 h时黄酮含量均超过CK。其中45 ℃处理白桦丛生苗4 h后,恢复培养6 h时黄酮含量最高,达到3.99 mg/g,比CK高32.14%。4 ℃处理白桦丛生苗4 h,在恢复培养48 h时,白桦丛生苗中的黄酮含量比CK提高38.37%。无论何种处理在恢复培养48 ~ 96 h,黄酮含量均呈下降趋势(图4C)。可见,不同温度胁迫下早期(6 ~ 48 h)对悬浮培养白桦丛生苗总黄酮积累最有利。
2.5 温度胁迫对白桦丛生苗抗逆酶活性的调节效应
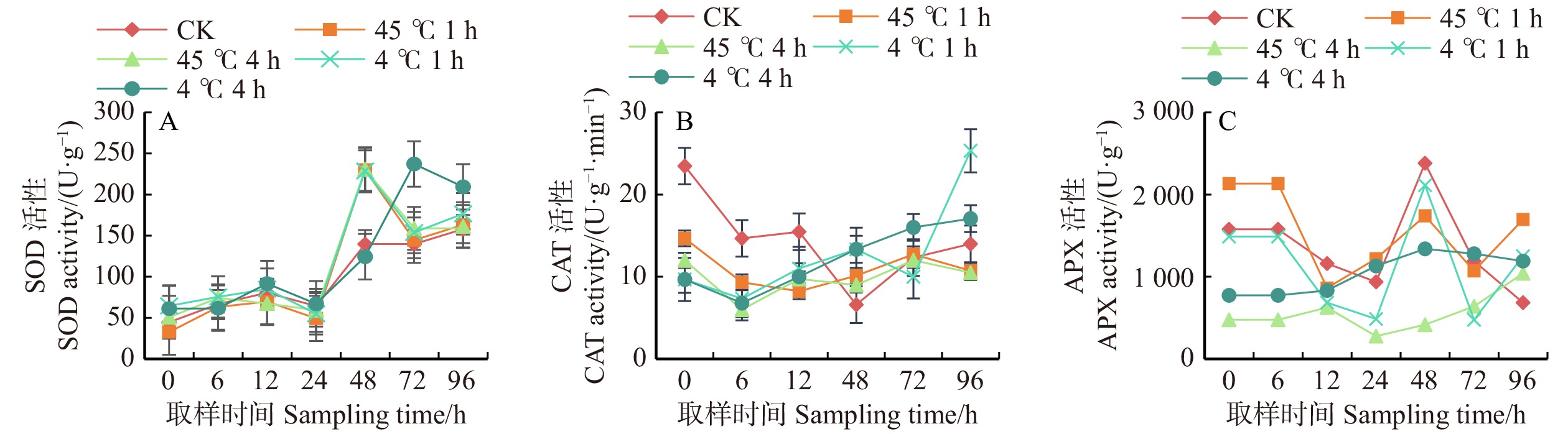
在恢复培养后0 ~ 24 h无论何种处理SOD活性与CK相比均无显著变化,24 h后SOD活性显著增强,除4 ℃胁迫白桦丛生苗1 h外,其余3种胁迫下在24 ~ 48 h SOD活性均比CK显著提高,而4 ℃胁迫4 h的白桦丛生苗在72 h活性最高,比CK提高69.77%。在恢复培养后期SOD活性均有降低(图5A)。
温度胁迫下白桦丛生苗CAT活性较稳定,在恢复培养0 ~ 12 h CAT活性均低于CK,48 h各处理较CK均有提高,但不显著。在恢复培养96 h,仅低温胁迫下处理的白桦丛生苗CAT活性高于CK,经低温处理1 h的丛生苗在96 h CAT活性达到最高峰,是CK的1.81倍。低温胁迫更有利于提高CAT活性(图5B)。
白桦组培苗热处理1 h后恢复培养0 h APX活性比CK高35.22%,其他处理活性均低于CK。在12、48 h各胁迫处理下APX活性均受抑制。仅在恢复培养96 h时各温度胁迫均有不同程度提高。上述分析结果表明,温度胁迫处理1 h更有利于APX活性的提高(图5C)。
温度胁迫对白桦丛生苗次生代谢产物含量与抗逆酶活性相关分析结果表明,温度胁迫处理1 h,白桦丛生苗总三萜、黄酮、齐墩果酸之间及其与抗逆酶活性的相关性不显著。分析可能是胁迫时间较短导致胁迫强度较低,无法使次生代谢物积累显著,防御酶活性显著增加。而在温度胁迫4 h后再恢复正常温度处理时各指标之间相关性显著提高,如45 ℃处理4 h下黄酮含量与APX酶、SOD与齐墩果酸含量均呈显著负相关(P < 0.05)(表2)。白桦丛生苗4 ℃处理4 h后恢复培养,总三萜与APX(P < 0.01)、CAT(P < 0.05)之间分别呈极显著正相关和显著正相关;黄酮与SOD活性呈显著负相关(P < 0.05);CAT与APX(P < 0.01)、SOD(P < 0.05)相关性达极显著和显著水平(表3),也说明3种防御酶协同发挥作用。
表 2 45 ℃胁迫4 h下白桦丛生苗次生产物含量与抗逆酶活性相关分析Table 2. Correlation analysis of secondary production content and anti-stress enzymeactivity of birch multiple shoots under 45 ℃ for 4 h项目
Item总三萜
Total triterpenoids齐墩果酸
Oleanolic acid黄酮
FlavonoidAPX SOD CAT 总三萜 Total triterpenoids 1.00 −0.55 0.25 −0.54 0.26 −0.02 齐墩果酸 Oleanolic acid 1.00 0.30 −0.10 −0.84* −0.41 黄酮 Flavonoid 1.00 −0.79* −0.24 −0.69 APX 1.00 0.30 0.08 SOD 1.00 0.43 CAT 1.00 注:*表示处理间显著相关(P < 0.05)。下同。Notes: * means significant correlation between treatments (P < 0.05). Same as below. 表 3 4 ℃胁迫4 h处理下白桦丛生苗次生产物含量与抗逆酶活性相关分析Table 3. Correlation analysis of secondary production content and anti-stress enzyme activity of birch multiple shoots at 4 ℃ for 4 h项目
Item总三萜
Total triterpenoids齐墩果酸
Oleanolic acid黄酮
FlavonoidAPX SOD CAT 总三萜 Total triterpenoids 1.00 0.57 −0.27 0.89** 0.49 0.81* 齐墩果酸 Oleanolic acid 1.00 0.21 0.35 0.17 0.51 黄酮 Flavonoid 1.00 −0.37 −0.80* −0.46 APX 1.00 0.69 0.96** SOD 1.00 0.77* CAT 1.00 注:**表示处理间极显著相关(P < 0.01)。Notes: ** means extremely significant correlation between treatments (P < 0.01). 3. 讨 论
丛生苗具有再生频率高、耗时短等优点,而广泛用于各种研究中。本研究确定附加2.0 mg/L 6-BA的NT固体培养基为白桦愈伤组织诱导形成丛生苗最佳培养基。此外,明确了1个培养周期中三萜积累动态,总三萜、齐墩果酸、黄酮分别在第10、15、20 天积累量最高。
本实验室前期完成了关于利用白桦细胞、植株进行三萜合成调控的大量研究,并取得一定进展[27-28]。马泓思等[27]利用MeJA处理白桦悬浮细胞,总三萜含量最高可达46.90 mg/g。本次研究利用白桦丛生苗悬浮培养,总三萜含量达到最高76.41 mg/g(10 d)。可见,以白桦丛生苗为试材相比白桦细胞悬浮培养能够获得更高含量的总三萜。苏欣等[28]以3年生白桦为试材,对白桦枝皮和叶进行检测,结果显示总三萜含量最高达168.17 mg/g,齐墩果酸含量最高达37.51 mg/g,明显高于本研究中丛生苗中三萜含量,但白桦丛生苗再生体系具有培养耗时短,再生频率高,产生的次生代谢产物稳定,不受季节、地域及虫害影响等优点,而白桦树木需要培育多年,才能获得较高含量的三萜物质。因此,白桦丛生苗的诱导及悬浮培养体系的建立无疑是有更广阔的应用前景。
温度胁迫诱导后植物细胞会在基因、酶和次生代谢水平上发生变化[29]。有研究发现,胡萝卜根中的萜类化合物含量随温度升高而升高。姜艳等[30]研究表明,低温可以通过诱导参与黄酮合成的酶的活性来增加植物中类黄酮的含量。人参三萜类皂苷生物合成途径中的关键酶达玛烯二醇合酶(dammarenediol synthase,DDS)使经过低温处理后其表达量迅速增加,进而提高人参皂苷产量[31-32]。
当植物面临胁迫时会产生过量的活性氧,对正常的细胞代谢造成干扰,同时激活植物体内的抗氧化系统,使得SOD、CAT、APX等抗氧化酶活性发生变化,进一步清除植物体内活性氧,从而抵御活性氧带来的损伤。抗氧化酶活性的提高可以增强植物体抵抗温度胁迫的能力[33-34]。SOD是能够将超氧阴离子自由基歧化生成H2O2的一种极其重要的抗氧化酶[35]。H2O2继续被CAT和APX催化,生成分子氧和水[36]。有研究表明SOD、APX、CAT等抗逆酶与次生代谢产物的产生有关。抗逆酶能够诱导植物激素及活性物质的生成,使植物体内源激素水平发生变化,进一步激活相关酶基因的表达,使关键酶被合成,进而调节次生代谢物积累[37]。同时,也有研究指出,在逆境胁迫下植物体内产生ROS,当植物体内含有较低的ROS时抗逆酶发挥作用。当植物体内的ROS含量过高,植物体内就会产生多种次生代谢产物对抗逆境胁迫[38]。
刘文盈[39]研究表明黑果枸杞(Lycium ruthenicum)愈伤组织通过CAT和APX活性变化迅速抵御低温伤害。Li等[40]将菠菜(Spinacia oleracea)进行热处理,发现APX活性增强。高慧如等[41]研究发现,黄酮类化合物与甘草抗氧化酶保护系统通过清除活性氧起到保护植物的作用。本研究通过不同温度胁迫处理白桦丛生苗,结果显示,温度胁迫可有效促进次生代谢产物合成。45 ℃处理白桦丛生苗1 h后,恢复培养48 h总三萜含量最高,为62.25 mg/g,比CK提高34.89%。45 ℃处理1 h的白桦丛生苗,在恢复培养72 h齐墩果酸含量最高,达2.33 mg/g,比对照高10.56倍。黄酮含量在45 ℃处理白桦丛生苗4 h后恢复培养6 h可达最高,为3.99 mg/g,比对照提高32.14%。同时发现,不同温度胁迫下白桦丛生苗3种抗逆酶活性高峰与变化趋势不同,说明SOD、CAT、APX 3种防御酶在抵御温度胁迫时相互协调共同发挥作用。防御酶活性与次生代谢产物含量相关分析表明:胁迫时间越长,防御酶活性与次生代谢物含量存在的相关性越显著。例如,45 ℃胁迫4 h,黄酮与APX呈显著负相关。4 ℃胁迫处理4 h总三萜含量与APX、CAT呈极显著或显著正相关。而两种温度胁迫1 h处理下相关性不显著。分析原因可能是胁迫时间过短,抗逆酶及次生产物合成没被充分激活,导致相关性不显著。
4. 结 论
本研究通过比较不同激素处理,确定从白桦愈伤组织到丛生苗最佳诱导培养基是附加2 mg/L 6-BA的NT固体培养基。提出了使用温度处理提高白桦丛生苗中总三萜、齐墩果酸和黄酮含量的方法。同时明确温度胁迫下SOD、CAT、APX 3种抗逆酶协调发挥作用共同参与次生产物合成。该研究方法无污染、低成本、可操作性强,效率高,为解决天然植物药物来源紧缺和工业化大规模生产白桦活性物质提供了一种新途径。
-
表 1 白桦愈伤组织不定芽分化
Table 1 Differentiation of adventitiousbuds from birch callus
处理
Treatment6-BA质量浓度
6-BA mass
concentration/
(mg·L−1)不定芽诱导率
Adventitious
bud induction
rate/%每块平均不定芽数
Average number
of adventitious
buds per pieceT2 1.0 46.67 3 T3 2.0 63.33 6 T4 5.0 53.33 4 表 2 45 ℃胁迫4 h下白桦丛生苗次生产物含量与抗逆酶活性相关分析
Table 2 Correlation analysis of secondary production content and anti-stress enzymeactivity of birch multiple shoots under 45 ℃ for 4 h
项目
Item总三萜
Total triterpenoids齐墩果酸
Oleanolic acid黄酮
FlavonoidAPX SOD CAT 总三萜 Total triterpenoids 1.00 −0.55 0.25 −0.54 0.26 −0.02 齐墩果酸 Oleanolic acid 1.00 0.30 −0.10 −0.84* −0.41 黄酮 Flavonoid 1.00 −0.79* −0.24 −0.69 APX 1.00 0.30 0.08 SOD 1.00 0.43 CAT 1.00 注:*表示处理间显著相关(P < 0.05)。下同。Notes: * means significant correlation between treatments (P < 0.05). Same as below. 表 3 4 ℃胁迫4 h处理下白桦丛生苗次生产物含量与抗逆酶活性相关分析
Table 3 Correlation analysis of secondary production content and anti-stress enzyme activity of birch multiple shoots at 4 ℃ for 4 h
项目
Item总三萜
Total triterpenoids齐墩果酸
Oleanolic acid黄酮
FlavonoidAPX SOD CAT 总三萜 Total triterpenoids 1.00 0.57 −0.27 0.89** 0.49 0.81* 齐墩果酸 Oleanolic acid 1.00 0.21 0.35 0.17 0.51 黄酮 Flavonoid 1.00 −0.37 −0.80* −0.46 APX 1.00 0.69 0.96** SOD 1.00 0.77* CAT 1.00 注:**表示处理间极显著相关(P < 0.01)。Notes: ** means extremely significant correlation between treatments (P < 0.01). -
[1] Wang S, Yang C, Zhao X, et al. Complete chloroplast genome sequence of Betula platyphylla: gene organization, RNA editing, and comparative and phylogenetic analyses[J/OL]. BMC Genomics, 2018, 19(1): 950 (2018−12−20) [2020−06−28]. https://doi.org/10.1186/s12864-018-5346-x.
[2] Malfa G A, Tomasello B, Acquaviva R, et al. Betula etnensis Raf. (Betulaceae) extract induced HO-1 expression and ferroptosis cell death in human colon cancer cells[J/OL]. International Journal of Molecular Sciences, 2019, 20(11): 2723 (2019−06−03) [2019−12−21]. https://doi.org/10.3390/ijms20112723.
[3] Ríos J L, Máñez S. New pharmacological opportunities for betulinic acid[J]. Planta Medica, 2018, 84(1): 8−19. doi: 10.1055/s-0043-123472
[4] Imran M, Rauf A, Abu-Izneid T, et al. Luteolin, a flavonoid, as an anticancer agent: a review[J/OL]. Biomedicine and Pharmacotherapy, 2019, 112: 108612 [2019−09−22]. https://doi.org/10.1016/j.biopha.2019.108612.
[5] 盛艳, 吴泽柱. 桦树汁营养成分及功能利用研究进展[J]. 农产品加工, 2017, 7(14):49−52. Sheng Y, Wu Z Z. Research progress in nutrient composition function and utilization of birch sap[J]. Farm Products Processing, 2017, 7(14): 49−52.
[6] Tan S N, Tee C S, Wong H L. Multiple shoot bud induction and plant regeneration studies of Pongamia pinnata[J]. Plant Biotechnology (Tokyo), 2018, 35(4): 325−334. doi: 10.5511/plantbiotechnology.18.0711a
[7] 王思瑶, 李明阳, 邵占媛, 等. 不同培养基及激素处理下柽柳丛生芽总酚、三萜及黄酮物质的积累[J]. 北京林业大学学报, 2014, 36(5):74−81. Wang S Y, Li M Y, Shao Z Y, et al. Accumulation of total phenolics, triterpenoids and flavonoids in tufted buds of Tamarix chinensis under different medium and hormone treatment[J]. Journal of Beijing Forestry University, 2014, 36(5): 74−81.
[8] 杨帆, 廉美兰, 李美兰, 等. 金线莲丛生芽培养及有效物质生产研究[J]. 中草药, 2016, 47(18):3284−3288. doi: 10.7501/j.issn.0253-2670.2016.18.024 Yang F, Lian M L, Li M L, et al. Multiple shoot culture and effective material production of Anoectochilus roxburghii[J]. Chinese Traditional and Herbal Drugs, 2016, 47(18): 3284−3288. doi: 10.7501/j.issn.0253-2670.2016.18.024
[9] 李球红, 宋华东, 周文怡, 等. 桂花幼胚离体培养形成丛生苗[J]. 杭州师范大学学报(自然科学版), 2015, 14(5):507−510. Li Q H, Song H D, Zhou W Y, et al. Multiple shoots formed from in vitro culture for young embryo of Osmanthus fragrans[J]. Journal of Hangzhou Normal University (Natural Science Edition), 2015, 14(5): 507−510.
[10] Gill S S, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants[J]. Plant Physiology and Biochemistry, 2010, 48(12): 909−930. doi: 10.1016/j.plaphy.2010.08.016
[11] Mittler R. ROS are good[J]. Trends in Plant Science, 2017, 22(1): 11−19. doi: 10.1016/j.tplants.2016.08.002
[12] 黄璐琦, 郭兰萍. 环境胁迫下次生代谢产物的积累及道地药材的形成[J]. 中国中药杂志, 2007, 32(4):277−280. doi: 10.3321/j.issn:1001-5302.2007.04.001 Huang L Q, Guo L P. Secondary metabolites accumulating and geoherbs formation under enviromental stress[J]. China Journal of Chinese Materia Medic, 2007, 32(4): 277−280. doi: 10.3321/j.issn:1001-5302.2007.04.001
[13] Mittler R, Vanderauwera S, Gollery M, et al. Reactive oxygen gene network of plants[J]. Trends in Plant Science, 2004, 9(10): 490−498. doi: 10.1016/j.tplants.2004.08.009
[14] 杜蕙, 王春明, 郭建国, 等. 葡萄生单轴霉菌对葡萄几种防御酶活性的影响[J]. 江苏农业科学, 2019, 47(15):151−154. Du H, Wang C M, Guo J G, et al. Effects of Plasmopara viticola on activity of defense-related enzymes in grape[J]. Jiangsu Agricultural Sciences, 2019, 47(15): 151−154.
[15] 郑金龙, 易克贤, 习金根, 等. 剑麻感染烟草疫霉菌后几种重要防御酶活性的变化[J]. 中国麻业科学, 2019, 41(5):210−216. doi: 10.3969/j.issn.1671-3532.2019.05.005 Zheng J L, Yi K X, Xi J G, et al. Several cases of sisal (H. 11648) infection with Phytophthora nicotianae changes in important defense enzyme activities[J]. Plant Fiber Sciences in China, 2019, 41(5): 210−216. doi: 10.3969/j.issn.1671-3532.2019.05.005
[16] Jumrani K, Bhatia V S. Interactive effect of temperature and water stress on physiological and biochemical processes in soybean[J]. Physiology Molecular Biology of Plants, 2019, 25(3): 671−681.
[17] 常志凯, 白祎杰, 董恒, 等. 高温胁迫诱导对白桦悬浮细胞三萜积累及防御酶活性的影响[J]. 生物技术通报, 2015, 31(9):111−118. Chang Z K, Bai Y J, Dong H, et al. The effects of high temperature stress on the accumulation of triterpenoid and the activity of defense enzyme in the suspension cells of birch[J]. Biotechnology Bulletin, 2015, 31(9): 111−118.
[18] Bhatia C, Pandey A, Gaddam S R, et al. Low Temperature-enhanced flavonol synthesis requires light-associated regulatory components in Arabidopsis thaliana[J]. Plant and Cell Physiology, 2018, 59(10): 2099−2112. doi: 10.1093/pcp/pcy132
[19] 刘佳, 全雪丽, 姜明亮, 等. 低温胁迫对人参皂苷生物合成途径基因家族表达特性的影响研究[J]. 中草药, 2016, 47(11):1956−1961. doi: 10.7501/j.issn.0253-2670.2016.11.024 Liu J, Quan X L, Jiang M L, et al. Effect of cold stress on expression characteristic of gene families of ginsenoside biosynthesis pathway[J]. Chinese Traditional and Herbal Drugs, 2016, 47(11): 1956−1961. doi: 10.7501/j.issn.0253-2670.2016.11.024
[20] 乌凤章, 王柏臣, 刘桂丰, 等. 低温胁迫对白桦幼苗生长和生理的影响[J]. 东北林业大学学报, 2008, 36(9):8−10. doi: 10.3969/j.issn.1000-5382.2008.09.003 Wu F Z, Wang B C, Liu G F, et al. Growth and physiological response of Betula platyphylla seedlings to low temperature stress[J]. Journal of Northeast Forestry University, 2008, 36(9): 8−10. doi: 10.3969/j.issn.1000-5382.2008.09.003
[21] 曲桂芹. 白桦热激反应的分子生态学研究[D]. 哈尔滨: 东北林业大学, 2001. Qu G Q. Study on molecular ecology of heat shock response in Betula platyphylla[D]. Harbin: Northeast Forestry Universiey, 2001.
[22] 王博. 促进白桦(Betula platyphylla)培养物中三萜物质积累的初步研究[D]. 哈尔滨: 东北林业大学, 2008. Wang B. Primary studies on aecumulation of triterpenoids in culture medium of Betula platyphylla[D]. Harbin: Northeast Forestry Universiey, 2008.
[23] 谭朝阳, 袁宏佳. HPLC法测定龙芽楤木药材中齐墩果酸的含量[J]. 中国中医药信息杂志, 2010, 17(1):46−47. doi: 10.3969/j.issn.1005-5304.2010.01.021 Tan C Y, Yuan H J. Determ
ination of oleanolic acid in cortex Aralia elatae by HPLC[J]. Chinese Journal of Information on Traditional Chinese Medicine, 2010, 17(1): 46−47. doi: 10.3969/j.issn.1005-5304.2010.01.021 [24] 施特尔马赫·B. 酶的测定方法[M]. 钱嘉渊, 译. 北京: 中国轻工业出版社, 1992. Stellmach B. Method for measuring enzyme[M]. Qian J Y, trans. Beijing: China Light Industry Press, 1992.
[25] 李合生. 植物生理生化实验原理和技术[M]. 北京: 高等教育出版社, 2000. Li H S. Principle and technology of plant physiological and biochemical experiments[M]. Beijing: Higher Education Press, 2000.
[26] 赵微. 温度及其与MeJA互作处理对白桦三萜合成的影响研究[D]. 哈尔滨: 东北林业大学, 2013. Zhao W. The effect of temperature and its interaction with MeJA treatment on the synthesis of triterpene in Betula platyphylla [D]. Harbin: Northeast Forestry University, 2013.
[27] 马泓思, 潘亚婕, 王艳, 等. Ca2+在介导MeJA诱导白桦悬浮培养三萜合成中的作用[J]. 植物研究, 2015, 35(1):117−126. doi: 10.7525/j.issn.1673-5102.2015.01.018 Ma H S, Pan Y J, Wang Y, et al. Effect of Ca2 + on mediating the MeJA-induced synthesis of triterpenoid in suspension cells of Betula platyphylla Suk.[J]. Bulletin of Botanical Research, 2015, 35(1): 117−126. doi: 10.7525/j.issn.1673-5102.2015.01.018
[28] 苏欣, 尹静, 孙建广, 等. 白桦植株中三萜前体及不同三萜分支产物积累特征[J]. 中草药, 2013, 44(24):3534−3539. Su X, Yin J, Sun J G, et al. Accumulative characteristics of triterpenoid precursor and different triterpenoid branch products in Betula platyphylla[J]. Chinese Traditional and Herbal Drugs, 2013, 44(24): 3534−3539.
[29] 毛美琴. 诱导子对朱砂根愈伤组织生长及三萜皂苷合成的影响[D]. 温江: 四川农业大学, 2018. Mao M Q. Effect of elicitor on callus growth and triterpenoid saponins synthesis in Ardisia crenata Sims allus[D]. Wenjiang: Sichuan Agricultural University, 2018.
[30] 姜艳, 刘奇, 冯尚国, 等. 环境因子对杭白菊黄酮类化合物和绿原酸含量的影响[J]. 中国现代应用药学, 2018, 35(2):225−230. Jiang Y, Liu Q, Feng S G, et al. Effects of environmental factors on the content of flavonoids and chlorogenic acid in Chrysanthemum morifolium Ramat[J]. Chinese Journal of Modern Applied Pharmacy, 2018, 35(2): 225−230.
[31] 林彦萍, 张美萍, 王康宇, 等. 人参皂苷生物合成研究进展[J]. 中国中药杂志, 2016, 41(23):4292−4302. Lin Y P, Zhang M P, Wang K Y, et al. Research achievements on ginsenosides biosynthesis from Panax ginseng[J]. China Journal of Chinese Materia Medica, 2016, 41(23): 4292−4302.
[32] 姜明亮. 低温对人参愈伤组织皂苷积累的影响[D]. 延边: 延边大学, 2016. Jiang M L. The effect of chilling on ginseoside accumulation in Panax ginseng cells[D]. Yanbian: Yanbian University, 2016.
[33] 杨颖丽, 吕丽荣, 徐玉玲, 等. 胞间活性氧产生对盐胁迫下两种小麦叶抗氧化反应的影响[J]. 兰州大学学报(自然科学版), 2019, 55(4):476−484. Yang Y L, Lü L R, Xu Y L, et al. Effects of apoplastic reactive oxygen species on the antioxidant responses in the leaves of two wheat seedlings under salt stress[J]. Journal of Lanzhou University (Natural Sciences), 2019, 55(4): 476−484.
[34] Reddy Y P, Yadav R K, Tripathi K, et al. Isolation and characterization of high temperature tolerant mutant from the cyanobacterium Anabaena doliolum[J]. Journal of Basic Microbiology, 2019, 59(3): 314−322. doi: 10.1002/jobm.201800447
[35] 赵远伟, 刘小京, 李存东, 等. 温度对盐胁迫小麦抗氧化机制的影响[J]. 中国生态农业学报, 2014, 22(12):1460−1468. Zhao Y W, Liu X J, Li C D, et al. Effect of temperature on antioxidation mechanism of wheat (Triticum aestivum L.) seedlings under salt stress[J]. Chinese Journal of Eco-Agriculture, 2014, 22(12): 1460−1468.
[36] Wu Z, Jiang Q, Yan T, et al. Ammonium nutrition mitigates cadmium toxicity in rice (Oryza sativa L.) through improving antioxidase system and the glutathione-ascorbate cycle efficiency[J/OL]. Ecotoxicology and Environmental Safety, 2020, 189: 110010 (2019−11−29) [2020−05−19]. https://doi.org/10.1016/j.ecoenv.2019.110010.
[37] 张涛. 人参及其皂苷生物合成对低温的生理生态响应机制研究[D]. 长春: 吉林农业大学, 2019. Zhang T. Physiological and ecoligical response mechanism of Panax ginseng and its saponins biosynthesis to low temperature[D]. Changchun: Jilin Agricultural University, 2019.
[38] 王斌. 干旱、H2O2及Na2S2O4对黄芩悬浮细胞代谢的影响[D]. 哈尔滨: 黑龙江中医药大学, 2020. Wang B. The effects of drought, H2O2 and Na2S2O4 stress on cell metabolism in Scutellaria baicalensis georgi suspension cells[D]. Harbin: Heilongjiang University of Chinese Medicine, 2020.
[39] 刘文盈. 低温胁迫对黑果枸杞愈伤组织的影响[J]. 基因组学与应用物, 2018, 37(1):408−412. Liu W Y. The effects of low temperature stress on the callus of Lycium ruthenicum Murr.[J]. Genomics and Applied Biology, 2018, 37(1): 408−412.
[40] Li S, Yu J, Li Y, et al. Heat-responsive proteomics of a heat-sensitive spinach variety[J/OL]. International Journal of Molecular Sciences, 2019, 20(16): 3872 (2019−08−08) [2020−07−05]. https://doi.org/10.3390/ijms20163872.
[41] 高慧如, 王佳慧, 关瑜, 等. 高温对甘草黄酮类成分的影响[J]. 中药材, 2019, 42(3):524−529. Gao H R, Wang J H, Guan Y, et al. Effect of high temperature on flavonoids of Glycyrrhiza uralensis[J]. Journal of Chinese Medicinal Materials, 2019, 42(3): 524−529.
-
期刊类型引用(3)
1. 赵常提,夏青霖,田地,陈冰瑞,朱瑞德,刘宵含,俞果,吉成均. 长期氮添加对温带落叶阔叶林优势植物叶片次生代谢产物的影响. 植物生态学报. 2024(12): 1576-1588 .  百度学术
百度学术
2. 申晨静,武文博,耿露冉,王福龙,赵鹏舟,宋金辉,詹亚光,尹静. 水杨酸、纳米氧化锌和促生真菌YZ13-1在水曲柳抵御干旱胁迫中的调控作用. 植物研究. 2023(03): 388-395 .  百度学术
百度学术
3. 王景哲,牛朝奎,梁馨元,申晨静,尹静. 水杨酸在白桦苗期抵御盐碱胁迫中的调控作用. 植物研究. 2023(03): 379-387 .  百度学术
百度学术
其他类型引用(8)



 下载:
下载: