Expression patterns and salt tolerance analysis of BpPAT1 gene in Betula platyphylla
-
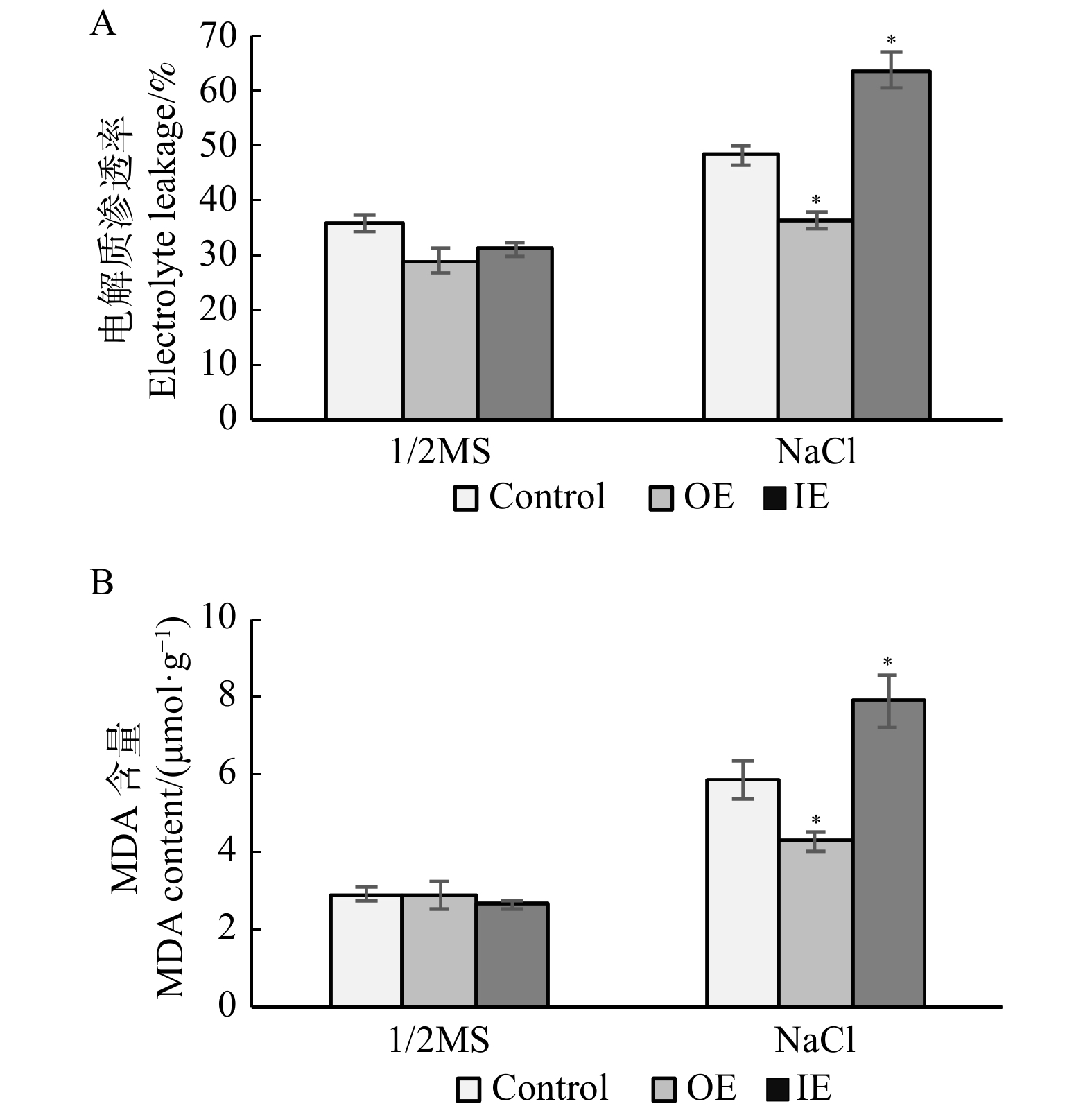
摘要:目的 GRAS家族是植物特有的具有高度保守羧基末端的转录因子家族,已有研究表明GRAS转录因子是植物胁迫反应中关键的转录调节因子之一。本研究拟对白桦中GRAS转录因子基因BpPAT1基因是否具有耐盐能力进行分析,为阐明白桦GRAS转录因子响应盐胁迫的分子调控机制奠定基础,进一步丰富木本植物GRAS转录因子响应逆境胁迫分子机制的研究。方法 从盐胁迫白桦转录组数据中筛选并获得了1条GRAS转录因子基因,将其命名为BpPAT1。利用蛋白多序列比对及系统进化树来分析BpPAT1与其他GRAS家族蛋白的亲缘关系。利用实时荧光定量PCR(qRT-PCR)技术分析盐胁迫及非胁迫条件下白桦根、茎和叶组织中BpPAT1的表达模式,初步鉴定其是否响应盐胁迫。为了进一步分析BpPAT1的抗逆功能,构建其植物过表达载体(pROKII-BpPAT1)与抑制表达载体(pFGC5941-BpPAT1),利用农杆菌介导的高效瞬时遗传转化体系,获得BpPAT1基因瞬时过表达、抑制表达及对照白桦植株。在盐胁迫下分别对BpPAT1瞬时表达及对照植株的耐盐相关生理指标进行测定,鉴定BpPAT1是否能调控白桦的耐盐能力。结果 多序列比对及系统进化树分析结果表明BpPAT1蛋白具有GRAS家族的序列特征,且与拟南芥中AtPAT1蛋白的亲缘关系较近。qRT-PCR结果表明:在盐胁迫6 h后,BpPAT1在白桦植株中的表达量显著上升(P < 0.05),说明该基因能响应盐胁迫。抗逆生理指标的测定结果表明:在白桦中过表达BpPAT1能够使过氧化物酶(POD)及超氧化物歧化酶(SOD)活性显著增强(P < 0.05),同时增加了白桦组织中的脯氨酸含量,降低了电解质渗透率及丙二醛含量。结论 白桦BpPAT1基因能响应盐胁迫,过表达BpPAT1显著增加了白桦POD、SOD酶活性和脯氨酸含量,降低了电解质渗透率及丙二醛含量,进而提高了ROS清除能力,有效增强了白桦的耐盐能力。Abstract:Objective GRAS family is a plant-specific transcription factor family, characterized by a highly conserved carboxyl terminus domain. Previous studies have shown that GRAS transcription factor is one of the key transcriptional regulators in plant stress response. The purpose of this study is to analyze the salt tolerance of GRAS transcription factor gene BpPAT1 gene in Betula platyphylla, so as to lay a foundation for elucidating the molecular regulation mechanism of GRAS transcription factor in response to salt stress. Our work enriched the research on the molecular mechanism of the GRAS transcription factors of woody plant in response to stress.Method In this study, one GRAS transcription factor gene was screened from the transcriptome data of B. platyphylla under salt stress and named as BpPAT1. Multiple sequence alignment and phylogenetic tree were used to analyze the genetic relationship between BpPAT1 and other organism’s GRAS family genes. Real-time fluorescence quantitative PCR (qRT-PCR) method was used to analyze the expression pattern of BpPAT1
in root, stem and leaf tissues of B. platyphylla under salt stress and normal condition, to identify whether it responded to salt stress or not. In order to further analyze the stress tolerance function of BpPAT1, plant overexpression vector (pROKII-BpPAT1) and inhibitory expression vector (pFGC5941-BpPAT1) were constructed. Transient overexpression and inhibitory expression of BpPAT1 gene and control B. platyphylla plants were obtained by Agrobacterium tumefaciens-mediated transient genetic transformation system. The physiological indexes related to salt tolerance were measured to identify whether the BpPAT1 was associated with salt tolerance in transient expression of BpPAT1 and control plants under salt stress. Result The results of multiple sequence alignment and phylogenetic tree analysis showed that BpPAT1 protein had the sequence characteristics of GRAS family and was closely related to AtPAT1 protein in A. thaliana. The result level of qRT-PCR showed that the expression of BpPAT increased significantly in B. platyphylla plants after 6 hours of salt stress, indicating that BpPAT1could respond to salt stress signal. The measurement results of the physiological indexes of stress resistance showed that the overexpression of BpPAT1 in B. platyphylla could significantly increase the activity of peroxidase (POD) and superoxide dismutase (SOD), increased the content of proline, and decreased electrolyte leakage and malondialdehyde (MDA) content. Conclusion The BpPAT1gene can respond to salt stress, overexpression of BpPAT1 significantly enhances POD, SOD enzyme activities and proline content, decreases electrolyte leakage and MDA content under salt stress, thus improves ROS scavenging ability and salt tolerance of B. platyphylla. -
Keywords:
- Betula platyphylla /
- GRAS transcription factor /
- BpPAT1 /
- gene expression /
- salt stress response
-
传统的树种分类主要根据形态学差异、杂交亲和力以及地理分布等特征开展[1]。但树木种群内极易发生变异,以及现在的人工杂交种众多,仅根据形态学特征进行分类已不能完全满足需要[2-4]。20世纪中叶,分子进化学被提出,这为生物进化的研究提供更可靠的方法。从核酸和蛋白序列比对分析生物进化原因和进化机制,可以更深层次探究生物进化的原因和关系,比较转录组、分子标记以及单基因克隆技术等都在植物分类和进化研究中被大量采用[5-10]。在转录组学中的应用主要是通过搜索和比较不同物种的同源基因,分析氨基酸或核苷酸替换数(Ka:非同义替换,Ks:同义替换)的差异,并建立不同物种间系统进化树,以分析各物种的进化地位[11]。但由于技术的限制,之前基于二代测序获取的序列读长较短,且拼接过程容易发生错误,很难准确预测直系同源基因全长。随着三代全长转录组测序技术的发展,这一问题迎刃而解,为不同物种进化分析提供了更完整和准确的序列[12-14]。
相对于比较转录组,通过克隆较短的DNA片段以比较物种的基因变异,具有高效快速等特点,被广泛用于鉴定物种亲缘关系。这种亲缘鉴定可分为核DNA(nrDNA)和叶绿体DNA(cpDNA)2种方法。cpDNA属于单亲遗传,研究cpDNA的变化可以反映种内和种间的差异,被前人广泛应用于物种亲缘鉴定[15],但cpDNA不能反映种间杂交和基因渐渗问题。而nrDNA进化速率较快,会发生基因重组,分析nrDNA可以探索物种内和物种间的基因传递,在物种分化和物种系统发育上也被广泛应用[16-18]。因此,结合nrDNA和cpDNA同时分析物种亲缘将提供更可靠的信息。
杨树(Populus spp.)作为林木研究的模式植物,具有生长快、适应性强、用途广等特点,被世界许多国家和地区广泛引种栽培,是人工用材林的重要树种[19]。我国具有丰富的杨树种质资源,有许多种为中国所特有,充分挖掘、开发和利用这些杨树资源,对培育杨树优良新品种具有重要意义。作为我国热带唯一的杨树树种,琼岛杨(P. qiongdaoensis)具有对热带高温高湿等不利于杨树生存环境的适应性[20]。了解物种的起源对于物种的适应性机制的挖掘及新品种的筛选和培育具有重要作用。因此,本研究基于琼岛杨对高温环境的独特适应性,通过高温胁迫处理,采用单分子实时和高通量测序技术比较了琼岛杨、加杨(P. canadensis)和小叶杨(P. simonii)的转录组差异,筛选直系同源基因,并结合nrDNA和cpDNA分析了琼岛杨与其他树种的亲缘关系及进化地位,为抗高温杨树新品种的开发及培育奠定了基础。
1. 材料与方法
1.1 试验材料
研究使用的加杨、琼岛杨和小叶杨幼苗被种植于海南大学的同一温室大棚中。筛选生长一致的幼苗用于对照组和热胁迫处理(40 ℃,1 h),然后收集它们的叶片进行RNA提取及质量检测,通过PacBio Sequel II系统测序获得了三代全长转录组序列,转录组数据已保存在中国科学院北京基因组研究所大数据中心(登录号:CRA002150,CRA002154和CRA002160)[21-23]。20个琼岛杨植株叶片采自其自然分布区域海南省白沙县黎族自治区青松乡。
1.2 DNA提取,PCR扩增及测序
DNA的提取采用传统CTAB法[24]。应用25 μL DNA聚合酶链式反应(PCR)体系,加入琼岛杨DNA 1 μL,正反向引物各1 μL(表1),引物序列来自之前的研究[25-27]、12.5 μL 2 × Taq PCR masterMix(购自TIANGEN公司)、9.5 μL ddH2O于94 ℃预变性3 min,94 ℃变性30 s, 55 ℃退火30 s,72 ℃延伸1 min,35个循环,最后72 ℃延伸5 min,4 ℃保存。PCR产物回收使用DNA纯化回收试剂盒(购自TIANGEN公司),然后送至广州天一辉公司使用Sanger法进行测序。
表 1 琼岛杨扩增序列引物信息Table 1. Amplified sequence primer information of P. qiongdaoensis基因名称
Gene name引物名称
Primer name引物序列
Primer sequenceUDP-SQ DSH3 F: TCTGCTTTCCACTTCTTGC DSH3 R: CATACTCTCCCATTGTCCC POPTRDRAFT_
575699DSH6 F: GCCTCCTGATTATTATGC DSH6 R: TATTACAAGCCCTTCCAG trnF trnL-trnF F: CGAAATTGGTAGACGCTACG trnL-trnF R: ATTTGAACTGGTGACACGAG atpⅠ atpⅠ-atpH F: CCAACCCAGCAGCAATAAC atpⅠ-atpH R: TATTTACAAGTGGTATTCAAGCT 1.3 转录本的注释及CDS预测
使用BLAST搜索NT数据库(e ≤ 10−10)[28-29]。使用Diamond BLASTX搜索NR、KOG、Swiss-Prot、GO和KEGG数据库(e ≤ 10−10)[30-34]。使用Hmmscan分析Pfam数据库[35]。
1.4 推定直系同源基因组中Ka/Ks值的估算
ANGEL软件被用来确定cDNA的蛋白编码序列(CDS),默认采用该软件容错模式。运用OrthoMCL算法对CDS序列进行直系同源基因识别和筛选[36]。采用paml-codeml计算Ks、Ka和Ka/Ks。利用公式T = K/2r估算杨树的分化时间[37],其中,K为遗传分歧,用Ks的平均值表示,r为双子叶植物同义替代率的平均值,为1.5 × 10−8年/位点[38-39]。
1.5 nrDNA和cpDNA基因序列获得与分析
克隆了20株琼岛杨和1株加杨的4个基因序列:糖生物合成酶(UDP-SQ)、POPTRDRAFT_575699、tRNA-Phe(trnF)和ATP合酶CF0A亚基(atpⅠ)基因,并结合从NCBI下载的5个杨树组(15个树种)和1个类外群组(3个树种)的4个基因序列进行分析(表2)。运用BioEdit V7.0.9软件对各基因序列进行多重比较、校对、剪切及拼接,并且对nrDNA的UDP-SQ和POPTRDRAFT_575699,以及cpDNA的trnF和atpⅠ序列进行组合[18]。然后使用MEGA 7.0对琼岛杨各基因及nrDNA和cpDNA组合的种内及种间遗传距离进行计算,分析各基因G+C平均含量、并使用最大似然法(maximum likelihood)和最小进化法(minimum evolution)构建系统发育树[3, 40]。
表 2 NCBI数据库获取的基因序列Table 2. Gene sequences obtained from NCBI database物种名称
Species name序列编号 GenBank atpⅠ trnF UDP-SQ POPTRDRAFT_575699 琼岛杨 Populus qiongdaoensis MW389752 ~ MW389771 MW389731 ~ MW389750 MW389689 ~ MW389708 MW389710 ~ MW389729 山杨 P. davidiana KF941071 KF940742 KF940382 KF940143 毛白杨 P. tomentosa KF941073 KF940744 KF940384 KF940145 响叶杨 P. adenopoda KF941089 KF940760 KF940400 KF940150 大叶杨 P. lasiocarpa KF941086 KF940757 KF940397 KF940158 异叶杨 P. heterophylla KX454634 KX454606 KX417462 KX417432 椅杨 P. wilsonii KX454638 KX454610 KX417466 KX417436 小叶杨 P. simonii KF941080 KF940751 KF940391 KF940152 苦杨 P. laurifolia KF941083 KF940754 KF940394 KF940155 毛果杨 P. trichocarpa KF941091 KF940762 KF940402 KF940163 加杨 P. canadensis MW389772 MW389751 MW389709 MW389730 黑杨 P. nigra KF941087 KF940758 KF940398 KF940159 阿富汗杨 P. afghanica KF941088 KF940759 KF940399 KF940160 美洲黑杨 P. deltoides KF941099 KF940770 KF940410 KF940171 灰胡杨 P. pruinosa KF941092 KF940763 KF940403 KF940164 胡杨 P. euphratica KF941096 KF940767 KF940407 KF940168 冬青叶杨 P. ilicifolia KX454633 KX454605 KX417461 KX417431 三蕊柳 Salix triandra KF941097 KF940768 KF940408 KF940169 钻天柳 S. arbutifolia KF941094 KF940765 KF940405 KF940166 大黄柳 S. raddeana KF941095 KF940766 KF940406 KF940167 2. 结果与分析
2.1 杨树基因注释
转录组测序分别获取了101 791、88 161和66 657个小叶杨、琼岛杨和加杨的全长转录本,平均长度分别为2 400、2 435和2 336 bp。加杨、琼岛杨和小叶杨分别有33 840、39 343和45 217个基因被注释,占比分别为50.77%、44.63%和44.42%(表3)。我们分别从3种杨树中随机选取1 000个转录本在NR数据库中比对,图1显示琼岛杨与毛果杨(P. trichocarpa)的比对率最高(53.38%),然后依次是胡杨(P. euphratica,22.63%)、毛白杨(P. tomentosa,2.84%)。加杨和小叶杨在毛果杨中的比对率也是最高,分别为59.48%和60.59%。
表 3 转录组数据注释结果Table 3. Annotation of transcriptome data注释数据库
Annotation database加杨
P. canadensis琼岛杨
P. qiongdaoensis小叶杨
P. simoniiNt 33 530 (50.30%) 38 920 (44.15%) 44 886 (44.10%) Nr 33 035 (49.56%) 38 338 (43.49%) 44 366 (43.59%) KEGG 32 611 (48.92%) 37 898 (42.99%) 43 820 (43.05%) Swiss-Prot 28 388 (42.59%) 32 860 (37.27%) 38 210 (37.54%) GO 22 121 (33.19%) 25 472 (28.89%) 30 206 (29.67%) Pfam 22 121 (33.19%) 25 472 (28.89%) 30 206 (29.67%) KOG 21 311 (31.97%) 24 422 (27.70%) 28 118 (27.62%) 合计 Total 33 840 (44.42%) 39 343 (44.63%) 45 217 (50.77%) 2.2 琼岛杨与其他两种杨树基因功能分类及直系同源基因分析
GO功能注释显示加杨、琼岛杨和小叶杨分别富集到257、322和216个功能组,3种杨树的生物过程富集的基因最多,多为代谢过程和细胞过程。琼岛杨与其他2种杨树基因的GO功能分类结果相差较大,琼岛杨与加杨中仅发现2个共同富集的功能组,分别是碳水化合物代谢过程和单一生物碳水化合物的代谢过程。
KEGG通路分析显示3种杨树的基因均参与了121个相同代谢通路,并且3种杨树各代谢通路中的基因占总基因的比例呈现一致性,加杨、琼岛杨和小叶杨中参与内质网中的蛋白质加工的基因最多,分别为834、709和831个基因(图2)。这显示了琼岛杨与其他2种杨树的基因在代谢过程中的功能具有相似性。
![]() 图 2 3种杨树基因功能分类横坐标为3种杨树富集基因最多的前20条KEGG通路。PPER. 内质网中的蛋白质加工;SP. 剪接体;PHST. 植物激素信号转导;RT. RNA转运;SSM. 淀粉和蔗糖代谢;EN. 胞吞;RI. 核糖体;PPI. 植物与病原体的相互作用;GL. 糖酵解/糖异生;MSP. mRNA监测途径;GDM. 乙醛酸和二羧酸酯代谢;OP. 氧化磷酸化;UMP. 泛素介导的蛋白水解;RD. RNA降解;CFPO. 光合生物中的碳固定;PM. 丙酮酸代谢;PE. 过氧化物;PH. 光合作用;PUM. 嘌呤代谢;ASNS. 氨基糖和核苷酸糖代谢。Abscissa is top 20 KEGG pathways with the most enriched genes in three Populus. PPER, protein processing in endoplasmic reticulum; SP, spliceosome; PHST, plant hormone signal transduction; RT, RNA transport; SSM, starch and sucrose metabolism; EN, endocytosis; RI, ribosome; PPI, plant-pathogen interaction; GL, glycolysis/gluconeogenesis; MSP, mRNA surveillance pathway; GDM, glyoxylate and dicarboxylate metabolism; OP, oxidative phosphorylation; UMP, ubiquitin mediated proteolysis; RD, RNA degradation; CFPO, carbon fixation in photosynthetic organisms; PM, pyruvate metabolism; PE, peroxisome; PH, photosynthesis; PUM, purine metabolism; ASNS, amino sugar and nucleotide sugar metabolism.Figure 2. Function classification in three Populus genes
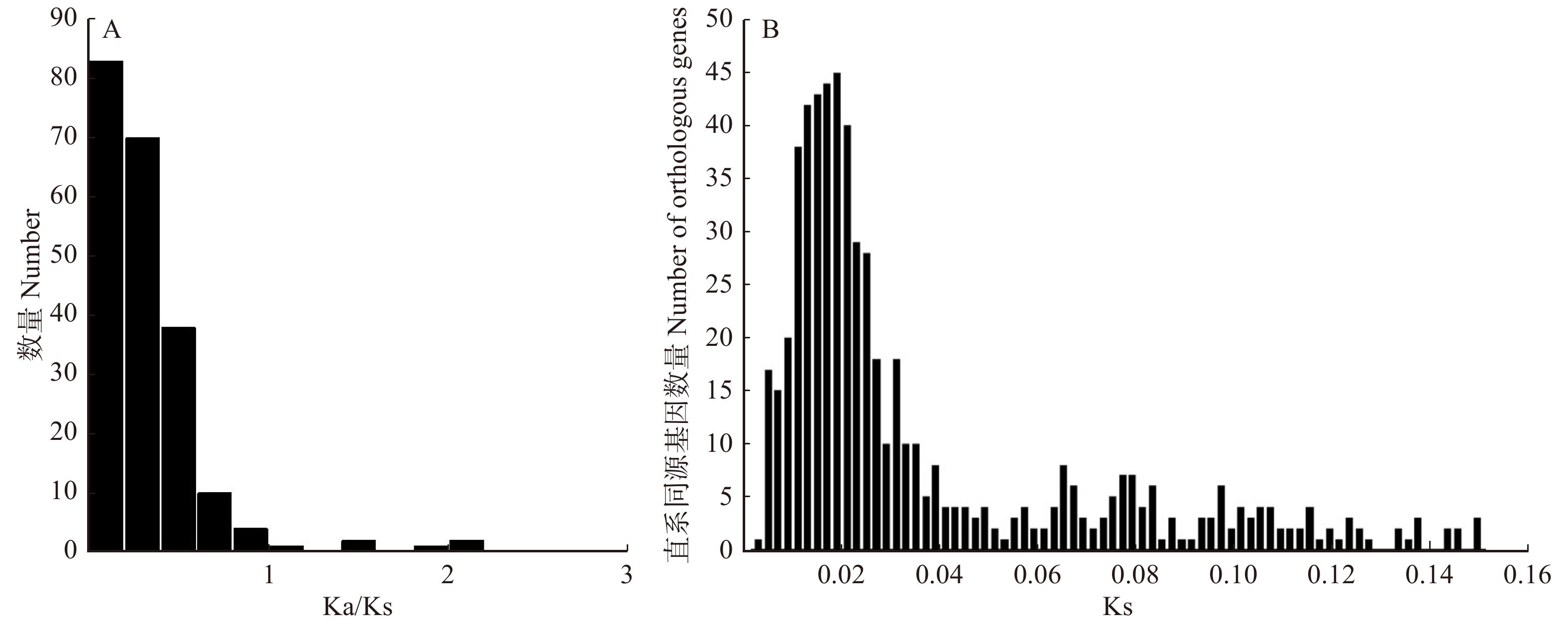
图 2 3种杨树基因功能分类横坐标为3种杨树富集基因最多的前20条KEGG通路。PPER. 内质网中的蛋白质加工;SP. 剪接体;PHST. 植物激素信号转导;RT. RNA转运;SSM. 淀粉和蔗糖代谢;EN. 胞吞;RI. 核糖体;PPI. 植物与病原体的相互作用;GL. 糖酵解/糖异生;MSP. mRNA监测途径;GDM. 乙醛酸和二羧酸酯代谢;OP. 氧化磷酸化;UMP. 泛素介导的蛋白水解;RD. RNA降解;CFPO. 光合生物中的碳固定;PM. 丙酮酸代谢;PE. 过氧化物;PH. 光合作用;PUM. 嘌呤代谢;ASNS. 氨基糖和核苷酸糖代谢。Abscissa is top 20 KEGG pathways with the most enriched genes in three Populus. PPER, protein processing in endoplasmic reticulum; SP, spliceosome; PHST, plant hormone signal transduction; RT, RNA transport; SSM, starch and sucrose metabolism; EN, endocytosis; RI, ribosome; PPI, plant-pathogen interaction; GL, glycolysis/gluconeogenesis; MSP, mRNA surveillance pathway; GDM, glyoxylate and dicarboxylate metabolism; OP, oxidative phosphorylation; UMP, ubiquitin mediated proteolysis; RD, RNA degradation; CFPO, carbon fixation in photosynthetic organisms; PM, pyruvate metabolism; PE, peroxisome; PH, photosynthesis; PUM, purine metabolism; ASNS, amino sugar and nucleotide sugar metabolism.Figure 2. Function classification in three Populus genes加杨、琼岛杨和小叶杨共识别660组直系同源基因,并计算了Ka、Ks和Ka/Ks平均值。如图3A所示,共有18个基因Ka/Ks > 1(2.73%),其中10个基因Ka/Ks > 2,大量基因Ka/Ks < 1(97.27%),这表明大量基因在杨树的进化过程中可能会进行纯化选择。
660组直系同源基因的Ks平均值为0.150 5,在0.02左右,直系同源基因数量达到峰值(图3B)。基于直系同源基因构建的进化树显示出琼岛杨与其他杨树具有明显差异(图4A),并估计了3种杨树分化时间大概为1.21 百万年前,该时间处于新生代的第四纪的更新世。为了更好的体现琼岛杨与其他杨树的亲缘关系,我们另外结合了毛果杨和簸箕柳(Salix suchowensis)搜索5个树种直系同源基因,共获得220组直系同源基因,并构建了5个物种进化树,图4B显示出毛果杨、加杨和小叶杨被聚为一个分支,琼岛杨和簸箕柳各为一分支。
![]() 图 4 琼岛杨系统进化树A.琼岛杨、加杨和小叶杨直系同源基因进化树;B.琼岛杨、加杨、小叶杨毛果杨和簸箕柳直系同源基因进化树。A, phylogenetic tree of orthologous genes of P. qiongdaoensis, P. canadensis and P. simonii; B, phylogenetic tree of orthologous genes of P. qiongdaoensis, P. canadensis, P. simonii, P. trichocarpa and Salix suchowensis.Figure 4. Phylogenetic tree of P. qiongdaoensis
图 4 琼岛杨系统进化树A.琼岛杨、加杨和小叶杨直系同源基因进化树;B.琼岛杨、加杨、小叶杨毛果杨和簸箕柳直系同源基因进化树。A, phylogenetic tree of orthologous genes of P. qiongdaoensis, P. canadensis and P. simonii; B, phylogenetic tree of orthologous genes of P. qiongdaoensis, P. canadensis, P. simonii, P. trichocarpa and Salix suchowensis.Figure 4. Phylogenetic tree of P. qiongdaoensis2.3 3种杨树直系同源基因差异表达模式分析
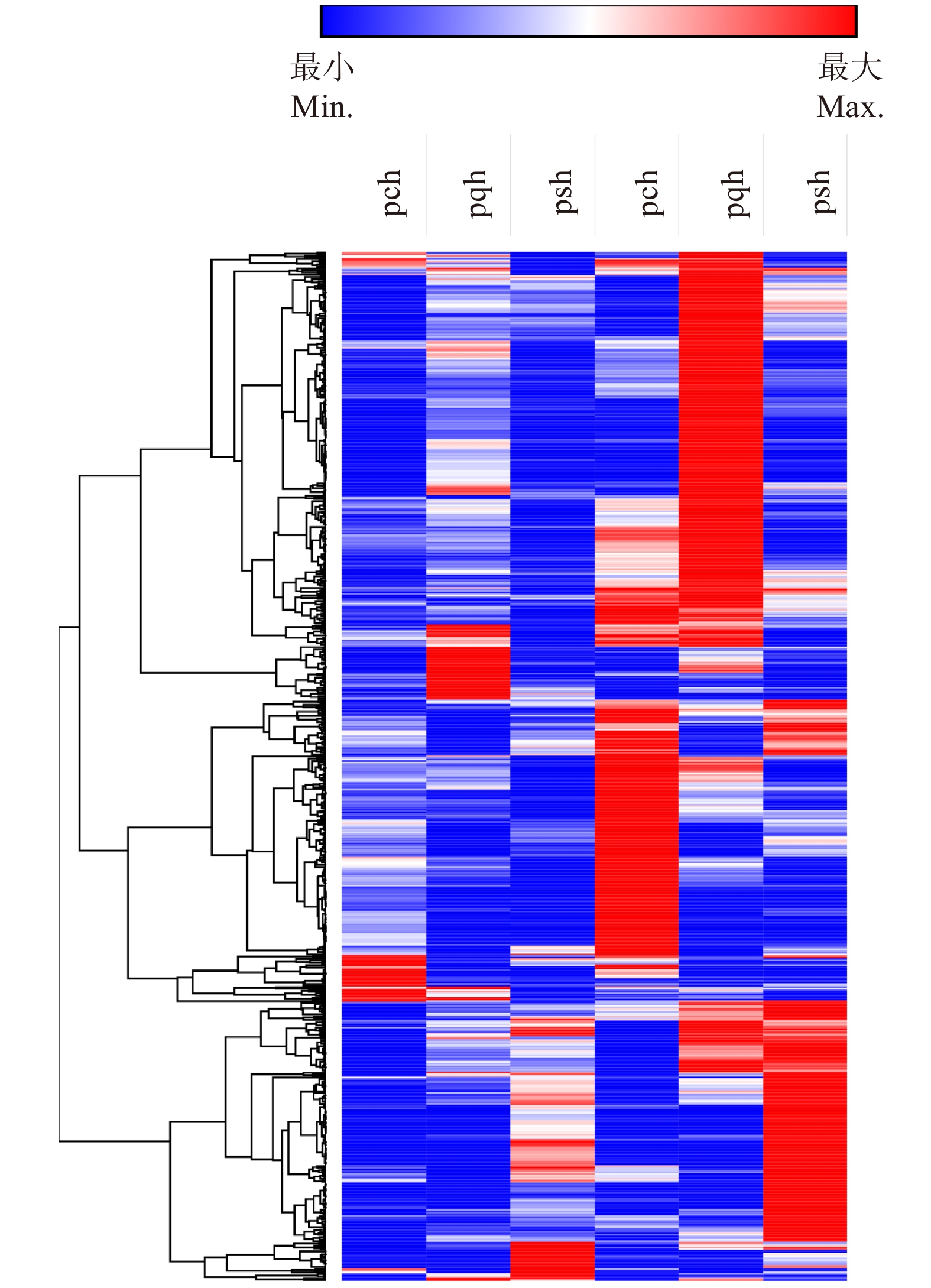
660组直系同源基因表达分析显示:尽管正常状态下,3种杨树基因表达模式具有差异,但在热胁迫下的基因表达模式很相似(图5)。660个直系同源基因组中,加杨有48个基因未表达,琼岛杨中136个基因未表达,小叶杨中142个未表达。加杨、琼岛杨和小叶杨中显著差异表达的基因分别为42、21和17个,其中4个同源基因在3种杨树中都显著差异表达,2个在琼岛杨和小叶杨中显著差异表达,5个在琼岛杨和加杨中都差异表达,且差异表达倍数相近(表4)。
![]() 图 5 3种杨树直系同源基因表达热图pch、pqh和psh分别为加杨、琼岛杨和小叶杨热胁迫处理;pcq、pqq和psq分别为加杨、琼岛杨和小叶杨对照组。The heat stress of P. canadensis, P. qiongdaoensis and P. simonii is named as pch, pqh and psh; the control of P. canadensis, P. qiongdaoensis and P. simonii is named as pcq, pqq and psq.Figure 5. Heat map of orthologus genes in three Populus species表 4 3种杨树差异表达直系同源基因Table 4. Differentially expression of orthologous genes in three Populus
图 5 3种杨树直系同源基因表达热图pch、pqh和psh分别为加杨、琼岛杨和小叶杨热胁迫处理;pcq、pqq和psq分别为加杨、琼岛杨和小叶杨对照组。The heat stress of P. canadensis, P. qiongdaoensis and P. simonii is named as pch, pqh and psh; the control of P. canadensis, P. qiongdaoensis and P. simonii is named as pcq, pqq and psq.Figure 5. Heat map of orthologus genes in three Populus species表 4 3种杨树差异表达直系同源基因Table 4. Differentially expression of orthologous genes in three Populus直系同源基因编号
Gene No. of orthologous gene琼岛杨 P. qiongdaoensis 加杨 P. canadensis 小叶杨 P. simonii 热处理
Heat stress对照
Controllog2FC 热处理
Heat stress对照
Controllog2FC 热处理
Heat stress对照
Controllog2FC OG07438 354.35 35.80 4.56 88.68 7.28 5.05 52.66 4.20 4.56 OG07244 188.23 4.62 6.66 1 329.18 31.22 6.85 103.81 1.39 7.16 OG09422 23.28 0.37 7.43 22.24 0.24 7.97 3.46 0.38 4.18 OG05822 3 381.21 2.03 12.19 318.20 0.48 10.80 370.24 0.11 12.65 OG07321 7.95 118.86 −2.97 6.49 115.96 −2.74 OG07470 75.24 13.14 3.82 17.13 3.28 3.84 OG07300 16.63 0.70 6.06 557.70 1.35 10.04 OG07384 279.51 11.27 6.08 583.29 20.48 6.31 OG09172 1 918.37 35.74 7.28 188.21 21.07 4.62 OG07291 113.69 13.64 4.39 242.96 4.12 6.94 OG07327 366.43 0.47 11.10 254.73 0.59 9.79 注:FC. 差异倍数。Note: FC, fold change. 2.4 琼岛杨cpDNA和nrDNA的序列多态性分析
为寻找琼岛杨更近的亲缘树种,我们采用单基因克隆的方法获得了琼岛杨atpⅠ、trnF、UDP-SQ和POPTRDRAFT_575699基因序列,基因长度分别为1 025、902、608和485 bp;G+C含量分别为29.56%、30.82%、45.23%和45.36%。2个cpDNA序列组合长度为1 927 bp,2个nrDNA序列组合长度为1 093 bp,G+C含量为30.15%和45.29%。nrDNA的G+C含量明显高于cpDNA。
atpⅠ、trnF、UDP-SQ和POPTRDRAFT_575699插入和缺失碱基数分别为27、18、3和0个(表5);单倍型数(单倍型多样性)分别为19(0.995)、18(0.989)、4(0.521)和2(0.100)个;多态位点分别为16、53、16和1个;突变总数分别为18、56、17和1个;简约位点分别为11、37、2和0个;单一位点分别为5、16、14和1个(表5)。综上所述,在琼岛杨中,cpDNA的序列多态性相对较高,进化较快,可提高种间鉴别能力,nrDNA的序列相对保守,进化相对较慢。
表 5 琼岛杨克隆基因序列长度及变异位点信息Table 5. Length and variant site information of amplified sequences of P. qiongdaoensis基因名称
Gene name长度
Length /bp插入/缺失个数
Number of the insertion
or deletion单倍型数
Number of
haplotype单倍型多样性
Haplotype
diversity多态位点
Polymorphic
site突变总数
Number of
mutation简约型位点
Parsimony
informative site单一位点
Single
siteatpⅠ 1 025 27 19 0.995 16 18 11 5 trnF 902 18 18 0.989 53 56 37 16 UDP-SQ 608 4 4 0.521 16 17 2 14 POPTRDRAFT_575699 485 2 2 0.100 1 1 0 1 2.5 nrDNA与cpDNA分析琼岛杨亲缘关系
利用遗传距离法计算琼岛杨组合基因序列cpDNA和nrDNA种内遗传距离以及与19种其他树种的种间遗传距离,结果显示cpDNA序列组合的种内和种间遗传距离分别为0 ~ 0.012和0.011 ~ 0.053,nrDNA序列组合分别为0 ~ 0.010和0.006 ~ 1.088。种间的遗传距离普遍大于种内遗传距离,表明种间的遗传分化明显大于种内的遗传分化,但也出现种间遗传距离小于种内遗传距离的情况,表明这些杨树与琼岛杨亲缘关系非常近。为了更准确地分析琼岛杨与其他树种的亲缘关系,进一步分析琼岛杨atpⅠ,trnF,UDP-SQ,POPTRDRAFT_575699以及组合基因序列cpDNA和nrDNA与其他树种的遗传距离(表6)。所有基因遗传距离的平均值显示出琼岛杨与白杨组亲缘最近,平均值为0.011,之后是青杨组(平均值0.015)和黑杨组(平均值0.016)。白杨组中,琼岛杨与响叶杨(P. adenopoda)遗传距离最近(0.010),毛白杨次之(0.011),最后是山杨(0.012),最大的山杨的平均遗传距离小于与其他派系杨树的遗传距离,显示了琼岛杨与白杨组具有最近亲缘关系。
表 6 琼岛杨与其他树种遗传距离Table 6. Genetic distance between P. qiongdaoensis and other Populus species物种名称
Species name分组
GroupnrDNA组合
nrDNA
combinationcpDNA组合
cpDNA
combinationUDP-SQ POPTRDRAFT_575699 atpⅠ trnF 平均值
Average各组平均值
Average of
each groupP. davidiana 白杨组
Leuce0.009 0.014 0.007 0.004 0.014 0.025 0.012 0.011 P. tomentosa 0.006 0.014 0.007 0.000 0.014 0.023 0.011 P. adenopoda 0.006 0.012 0.007 0.000 0.013 0.023 0.010 P. lasiocarpa 大叶杨组
Leucoides0.013 0.013 0.007 0.007 0.014 0.025 0.013 0.388 P. heterophylla 1.073 0.016 0.013 1.158 0.017 1.169 0.575 P. wilsonii 1.064 0.016 0.009 1.160 0.017 1.193 0.577 P. simonii 青杨组
Tacamahaca0.015 0.013 0.009 0.007 0.014 0.026 0.014 0.015 P. laurifolia 0.015 0.017 0.009 0.007 0.018 0.032 0.016 P. trichocarpa 0.009 0.017 0.009 0.002 0.017 0.029 0.014 P. nigra 黑杨组
Aigeiros0.020 0.011 0.011 0.011 0.012 0.023 0.015 0.016 P. afghanica 0.018 0.014 0.011 0.009 0.014 0.026 0.015 P. canadensis 0.018 0.016 0.010 0.008 0.022 0.026 0.017 P. deltoides 0.018 0.016 0.011 0.009 0.017 0.029 0.017 P. pruinosa 胡杨组
Turanga0.013 0.018 0.013 0.002 0.020 0.027 0.016 0.205 P. euphratica 0.016 0.018 0.015 0.004 0.020 0.027 0.017 P. ilicifolia 1.088 0.018 0.017 1.159 0.020 1.195 0.583 S. triandra 外类群
Outgroup0.083 0.053 0.049 0.040 0.053 0.062 0.057 0.056 S. arbutifolia 0.083 0.052 0.053 0.036 0.051 0.062 0.056 S. raddeana 0.081 0.053 0.047 0.038 0.052 0.062 0.056 我们使用了最大似然法和最小进化法分别构建所有基因及组合基因的系统进化树,所有基因通过两种方法构建的进化树均把琼岛杨聚到一起。我们展示了最大似然法构建的进化树,cpDNA构建的进化树显示琼岛杨与白杨组具有最近亲缘关系,且置信度均超过50,trnF中置信度50,atpⅠ中置信度58,cpDNA序列组合置信度为67(图6A)。nrDNA构建的进化树显示琼岛杨与白杨组也具有较近亲缘关系,置信度均小于50(图6B),UDP-SQ中置信度为15,POPTRDRAFT_575699中置信度为40,且均把类外群分到杨树大分支,显示出nrDNA序列对于琼岛杨与其他物种间的区分度较差。
3. 结论与讨论
鉴定物种的亲缘关系,通过直系同源基因的比较是最直接有效的方法[41]。通过搜索我们获取的转录本数据,加杨、琼岛杨和小叶杨一共获取了660组直系同源基因。计算物种的直系同源基因的Ka、Ks及Ka/Ks值可用于评估物种的亲缘关系、计算分化时间及研究进化过程中受正选择的基因[42-43]。本研究中3个物种的直系同源基因的Ks值平均值为0.150 5,峰值为0.02,Ks值水平相对较低,Ka/Ks < 1占比97.27%,表明3种杨树具有较近亲缘关系。构建的系统发育树也显示琼岛杨与加杨和小叶杨具有较近亲缘关系,但琼岛杨更为进化,并通过T = K/2r估算出琼岛杨与这2个物种的分化时间大概为1.21 百万年前左右的更新世[44]。不同物种相同处理条件下同类基因的表达模式也能反映物种的亲缘关系。在我们研究中,660组直系同源基因表达量分析显示:尽管正常状态下,3种杨树大量的基因表达模式存在差异,但在热胁迫下它们的表达模式却很相似,进一步表明了琼岛杨、加杨和小叶杨具有较近亲缘关系。另外,我们结合毛果杨和簸箕柳搜索了5个树种直系同源基因共220组,基于这些直系同源基因构建了5个树种的进化树,结果显示:加杨、小叶杨和毛果杨被聚为同一分支,琼岛杨和簸箕柳各为一个支。同时,琼岛杨、加杨和小叶杨的转录本与毛果杨的比对率均为最高,说明了这些杨树具有较近亲缘关系,但琼岛杨与其他杨树间具有一定差异。
我们进一步通过单基因克隆的方法分析了琼岛杨在杨属中的进化地位,该技术是用于物种亲缘关系分析的重要方法之一[45-47]。我们在20株琼岛杨上克隆了4个基因序列,通过琼岛杨种内的分析显示atpⅠ和trnF多态位点分别为16和53,明显高于nrDNA的UDP-SQ(16)和POPTRDRAFT_575699(1),这表明cpDNA相对于nrDNA可能进化速率更快,在琼岛杨中cpDNA可能具有更好的物种鉴别能力。另外,为了分析琼岛杨的亲缘关系,我们结合19个树种进行了序列多重比较,通过遗传距离法计算atpⅠ、trnF、UDP-SQ、POPTRDRAFT_575699以及组合基因序列cpDNA和nrDNA与其他树种的遗传距离,平均遗传距离(0.011)均显示琼岛杨与白杨组亲缘最近,这与Wang等[48]的研究一致。使用最大似然法和最小进化法构建的所有基因的系统进化树均把琼岛杨聚到一起,表明该方法适用于琼岛杨亲缘关系分析。其中,cpDNA和nrDNA组合序列构建的进化树均显示琼岛杨与白杨组具有最近亲缘关系,遗传距离和构建的进化树结果表明:琼岛杨确实与白杨组亲缘关系最近。另外,cpDNA序列组合的进化分支置信度明显高于nrDNA序列组合,综上结果表明:在琼岛杨中,cpDNA基因比nrDNA基因更适合用于物种鉴别。
-
图 1 白桦BpPAT1蛋白的多序列比对分析(A)及系统进化树分析(B)
AtPAT1. 拟南芥(NP_001332482.1);QsGRAS. 欧洲栓皮栎(XP_023916980.1);MrGRAS. 杨梅(KAB1208676.1);JrGRAS. 胡桃(XP_018849898.1);VvGRAS. 葡萄(XP_002272334.1);JcGRAS20. 麻风树(XP_012081428.1);CfGRAS. 土瓶草(GAV74587.1);DlGRAS54. 龙眼(AGE44291.1);TcGRAS. 可可(EOX93442.1)。AtPAT1, Arabidopsis thaliana (NP_001332482.1); QsGRAS, Quercus suber (XP_023916980.1); MrGRAS, Morella rubra (KAB1208676.1); JrGRAS, Juglans regia (XP_018849898.1);VvGRAS, Vitis vinifera (XP_002272334.1); JcGRAS20, Jatropha curcas (XP_012081428.1); CfGRAS, Cephalotus follicularis (GAV74587.1); DlGRAS54, Dimocarpus longan (AGE44291.1); TcGRAS, Theobroma cacao (EOX93442.1).
Figure 1. Multiple sequence alignment analysis of Betula platyphylla BpPAT1 protein (A) and phylogenetic tree analysis (B)
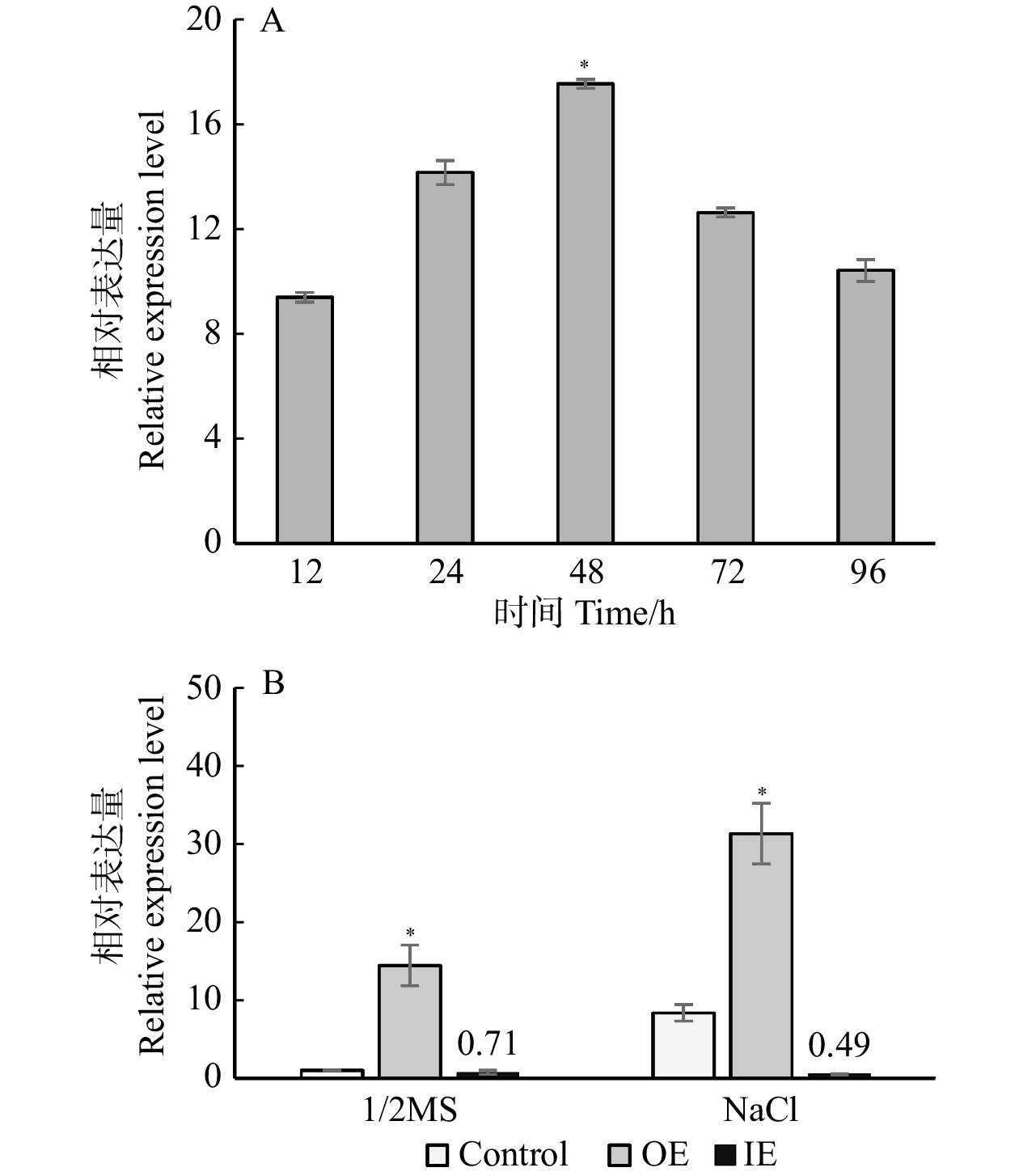
图 3 盐胁迫下BpPAT1基因在瞬时表达白桦植株中的表达情况
A. 盐胁迫下不同转化时间对照植株及瞬时过表达植株BpPAT1基因的表达水平;B. 正常条件下与盐胁迫后对照植株及瞬时转化植株BpPAT1基因的表达水平;Control. 对照植株;OE. 瞬时过表达植株;IE. 瞬时抑制表达植株;*. 显著性差异(P < 0.05)。下同。A, expression levels of BpPAT1 gene in control plants and transient overexpressed plants at different transformation time under salt stress; B, expression level of BpPAT1 gene in control plants and transient transformed plants under normal conditions and salt stress. Control, control plant; OE, transient overexpression plant; IE, transient inhibitory expression plant. Asterisks indicate significant difference (P < 0.05). The same below.
Figure 3. Expression of BpPAT1 gene in transient expression plants of B. platyphylla under salt stress
表 1 引物序列
Table 1 Primers used in this study
用途 Application 引物名称 Primer name 引物序列(5′—3′) Primer sequence (5′−3′) 实时荧光定量 PCR Quantitative real-time PCR q-BpPAT1-F TACTGCTGCATTCTATCCAC q-BpPAT1-R ACTTACCAAGAAGCTCATG q-BpPAT1-OE-F CCCCACATCCGCATAACA q-BpPAT1-OE-R CCCAGGTCGAATCCCAAG q-BpPAT1-IE-F ACGAACGGTGTTGCACTT q-BpPAT1-IE-R GAGCCATAGGTATTGTCAGG Actin-F TGAGAAGAGCTATGAGTTGC Actin-R GTAGATCCACCACTAAGCAC Tubulin-F TCAACCGCCTTGTCTCTCAGG Tubulin-R TGGCTCGAATGCACTGTTGG 基因克隆 Gene cloning BpPAT1-F GCTCTAGAATGTCCAACGGATTGTACTATC BpPAT1-R GGGTACCTCACTTCCATGCACAAGCAG 载体构建 Vector verification pROKⅡ-F AGACGTTCCAACCACGTCTT pROKⅡ-R CCAGTGAATTCCCGATCTAG pFGC5941-cisF CGCTCGAGTATAAGAGCT pFGC5941-cisR ACCTTCCCACAATTCGTCGG pFGC5941-antiF GCATGCTATGCATTCAAT pFGC5941-antiR CGTGCACAACAGAATTGAAAGC pFGC5941-BpPAT1-cisF CCCATGGCAGCTATGCTACAATGATAG pFGC5941-BpPAT1-cisR TTGGCGCGCCTCCACATATAGAAGAGCCAT pFGC5941-BpPAT1-antiF CTCTAGACAGCTATGCTACAATGATAG pFGC5941-BpPAT1-antiR CGGATCCTCCACATATAGAAGAGCCAT -
[1] 刘中原, 刘峥, 徐颖, 等. 白桦HSFA4转录因子的克隆及耐盐功能分析[J]. 林业科学, 2020, 56(5):69−79. doi: 10.11707/j.1001-7488.20200508 Liu Z Y, Liu Z, Xu Y, et al. Cloning and salt tolerance analysis of transcription factor HSFA4 from Betula platyphylla[J]. Scientia Silvae Sinicae, 2020, 56(5): 69−79. doi: 10.11707/j.1001-7488.20200508
[2] 刘强, 张贵友, 陈受宜. 植物转录因子的结构与调控作用[J]. 科学通报, 2000, 45(14):1465−1474. doi: 10.3321/j.issn:0023-074X.2000.14.002 Liu Q, Zhang G Y, Chen S Y. The structure and regulation of plant transcription factors[J]. Chinese Science Bulletin, 2000, 45(14): 1465−1474. doi: 10.3321/j.issn:0023-074X.2000.14.002
[3] 唐瑞, 韩妮, 虎亚静, 等. 黄瓜GRAS家族全基因组鉴定与表达分析[J/OL]. 分子植物育种, 2021, 19(13): 4242−4251 [2020−07−23]. http://kns.cnki.net/kcms/detail/46.1068.S.20200526.1639.012.html. Tang R, Han N, Hu Y J, et al. Genome-wide identification and expression analysis of GRAS genes in Cucumber[J/OL]. Molecular Plant Breeding, 2021, 19(13): 4242−4251 [2020−07−23]. http://kns.cnki.net/kcms/detail/46.1068.S.20200526.1639.012.html.
[4] Lee M H, Kim B, Song S K, et al. Large-scale analysis of the GRAS gene family in Arabidopsis thaliana[J]. Plant Molecular Biology, 2008, 67(6): 659−670. doi: 10.1007/s11103-008-9345-1
[5] Tian C, Wan P, Sun S, et al. Genome-wide analysis of the GRAS gene family in rice and Arabidopsis[J]. Plant Molecular Biology, 2004, 54(4): 519−532. doi: 10.1023/B:PLAN.0000038256.89809.57
[6] Song X M, Liu T K, Duan W K, et al. Genome-wide analysis of the GRAS gene family in Chinese cabbage (Brassica rapa ssp. pekinensis)[J]. Genomics, 2013, 103(1): 135−146.
[7] Cordelia B. The role of GRAS proteins in plant signal transduction and development[J]. Planta, 2004, 218(5): 683−692. doi: 10.1007/s00425-004-1203-z
[8] Liu Y D, Huang W, Xian Z Q, et al. Overexpression of SlGRAS40 in tomato enhances tolerance to abiotic stresses and influences auxin and gibberellin signaling[J/OL]. Frontiers in Plant Science, 2017, 8: 1659 [2020−09−23]. https://doi.org/10.3389/fpls.2017.01659.
[9] Kim Y J, Yang D H, Park M Y, et al. Overexpression of zoysia ZjCIGR1 gene confers cold stress resistance to zoysiagrass[J]. Plant Biotechnology Reports, 2020, 14(1): 21−31. doi: 10.1007/s11816-019-00570-z
[10] Laura D L, Joanna W D, Jocelyn E M, et al. The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root[J]. Cell, 1996, 86(3): 423. doi: 10.1016/S0092-8674(00)80115-4
[11] Helariutta Y, Fukaki H, Wysocka D J, et al. The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling[J]. Cell, 2000, 101(5): 555−567. doi: 10.1016/S0092-8674(00)80865-X
[12] Chen K M, Li H W, Chen Y F, et al. TaSCL14, a novel wheat (Triticum aestivum L.) GRAS gene, regulates plant growth, photosynthesis, tolerance to photooxidative stress, and senescence[J]. Journal of Genetics and Genomics, 2015, 42(1): 21−32. doi: 10.1016/j.jgg.2014.11.002
[13] Torres-Galea P, Huang L F, Chua N H, et al. The GRAS protein SCL13 is a positive regulator of phytochrome-dependent red light signaling, but can also modulate phytochrome a responses[J]. Molecular Genetics & Genomics, 2006, 276(1): 13−30.
[14] 吴捷, 兰士波, 宁晓光. 东北白桦种质资源生态耦合性分析及可持续利用策略[J]. 林业勘查设计, 2017(4):62−64. doi: 10.3969/j.issn.1673-4505.2017.04.029 Wu J, Lan S B, Ning X G. Ecology coupling analysis and sustainable utilization strategy of Betula platyphylla germplasm resource[J]. Forest Investigation Design, 2017(4): 62−64. doi: 10.3969/j.issn.1673-4505.2017.04.029
[15] Liu X, Widmer A. Genome-wide comparative analysis of the GRAS gene family in Populus, Arabidopsis and rice[J]. Plant Molecular Biology Reporter, 2014, 32(6): 1129−1145. doi: 10.1007/s11105-014-0721-5
[16] Li Z, Lu H, He Z, et al. Selection of appropriate reference genes for quantitative real-time reverse transcription PCR in Betula platyphylla under salt and osmotic stress conditions[J/OL]. PLoS One, 2019, 14(12): e0225926 [2020−08−03]. https://doi.org/10.1371/journal.pone.0225926.
[17] Livak K, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method[J]. Methods, 2001, 25(4): 402−408. doi: 10.1006/meth.2001.1262
[18] 郭勇, 王玉成, 王智博. 一种基于农杆菌介导的拟南芥瞬时转化技术优化[J]. 东北林业大学学报, 2016, 44(6):41−44, 83. doi: 10.3969/j.issn.1000-5382.2016.06.011 Guo Y, Wang Y C, Wang Z B. Optimizing transient genetic transformation method on Arabidopsis plants mediated by Agrobacterium tumefaciens[J]. Journal of Northeast Forestry University, 2016, 44(6): 41−44, 83. doi: 10.3969/j.issn.1000-5382.2016.06.011
[19] 王关林, 方宏筠. 植物基因工程实验技术指南[M]. 北京: 科学出版社, 2016. Wang G L, Fang H J. Laboratory guide for plant genetic engineering[M]. Beijing: Science Press, 2016.
[20] 聂显光. 柽柳ThbHLH1基因调控抗逆响应的分子机理研究[D]. 哈尔滨: 东北林业大学, 2014. Nie X G. Functional characterization of the abiotic stress response mechanisms of ThbHLH1 transcript factor from Tamarix hispida[D]. Harbin: Northeast Forestry University, 2014.
[21] 卢惠君, 李子义, 梁瀚予, 等. 刚毛柽柳NAC24基因的表达及抗逆功能分析[J]. 林业科学, 2019, 55(3): 54−63. Lu H J, Li Z Y, Liang H Y, et al. Expression and stress tolerance analysis of NAC24 from Tamarix hispida[J]. Scientia Silvae Sinicae, 2019, 55(3): 54−63.
[22] 刘羽佳. 拟南芥AtbHLH112基因调控植物抗逆机制的研究[D]. 哈尔滨: 东北林业大学, 2013. Liu Y J. Study on stress tolerance mechanism mediated by AtbHLH112 from Arabidopsis thaliana[D]. Harbin: Northeast Forestry University, 2013.
[23] 国会艳. 白桦BplMYB46基因调控抗旱耐盐和次生壁形成的分子机理[D]. 哈尔滨: 东北林业大学, 2014. Guo H Y. The molecular mechanism of BplMYB46 from Betula platyphylla in mediating drought and salt tolerance and formation of secondary wall[D]. Harbin: Northeast Forestry University, 2014.
[24] Lu X, Liu W, Xiang C, et al. Genome-wide characterization of GRAS family and their potential roles in cold tolerance of Cucumber (Cucumis sativus L.)[J/OL]. International Journal of Molecular Sciences, 2020, 21(11): 3857 [2020−08−29]. https://doi.org/10.3390/ijms21113857.
[25] Sidhu N S, Pruthi G, Singh S, et al. Genome-wide identification and analysis of GRAS transcription factors in the bottle gourd genome[J]. Scientific Reports, 2020, 10(1): 2367−2372. doi: 10.1038/s41598-020-59417-1
[26] Ma H S, Liang D, Shuai P, et al. The salt- and drought-inducible poplar GRAS protein SCL7 confers salt and drought tolerance in Arabidopsis thaliana[J]. Journal of Experimental Botany, 2010, 61(14): 4011−4019. doi: 10.1093/jxb/erq217
[27] 周莲洁, 杨中敏, 张富春, 等. 新疆盐穗木GRAS转录因子基因克隆及表达分析[J]. 西北植物学报, 2013, 33(6):1091−1097. doi: 10.3969/j.issn.1000-4025.2013.06.004 Zhou L J, Yang Z M, Zhang F C, et al. Expression analysis and cloning of GRAS transcription factor gene from Halostachys capsica[J]. Acta Botanica Boreali-Occidentalia Sinica, 2013, 33(6): 1091−1097. doi: 10.3969/j.issn.1000-4025.2013.06.004
[28] Liu Z Y, Wang P L, Zhang T Q, et al. Comprehensive analysis of BpHSP genes and their expression under heat stresses in Betula platyphylla[J]. Environmental and Experimental Botany, 2018, 152: 167−176. doi: 10.1016/j.envexpbot.2018.04.011
[29] Zang D D, Wang C, Ji X Y, et al. Tamarix hispida zinc finger protein ThZFP1 participates in salt and osmotic stress tolerance by increasing proline content and SOD and POD activities[J]. Plant Science, 2015, 235: 111−121. doi: 10.1016/j.plantsci.2015.02.016
[30] Yang G Y, Yu L L, Zhang K M, et al. A ThDREB gene from Tamarix hispida improved the salt and drought tolerance of transgenic tobacco and T. hispida[J]. Plant Physiology & Biochemistry, 2017, 113: 187−197.
[31] Li P, Zhang B, Su T B, et al. BrLAS, a GRAS transcription factor from Brassica rapa, is involved in drought stress tolerance in transgenic Arabidopsis[J/OL]. Frontiers in Plant Science, 2018(9): 1792 [2020−08−06]. https://doi.org/10.3389/fpls.2018.01792.
[32] Guo H Y, Wang Y C, Wang L Q, et al. Expression of the MYB transcription factor gene BplMYB46 affects abiotic stress tolerance and secondary cell wall deposition in Betula platyphylla[J]. Plant Biotechnology Journal, 2017, 15(1): 107−121. doi: 10.1111/pbi.12595
[33] He Z H, Li Z Y, Lu H J, et al. The NAC protein from Tamarix hispida, ThNAC7, confers salt and osmotic stress tolerance by increasing reactive oxygen species scavenging capability[J/OL]. Plants, 2019, 8(7): 221 [2020−07−12]. https://doi.org/10.3390/plants8070221.
[34] Yuan Y Y, Fang L C, Sospeter K K, et al. Overexpression of VaPAT1, a GRAS transcription factor from Vitis amurensis, confers abiotic stress tolerance in Arabidopsis[J]. Plant Cell Reports, 2016, 35(3): 655−666. doi: 10.1007/s00299-015-1910-x
[35] Zhang S, Li X, Fan S, et al. Overexpression of HcSCL13, a Halostachys caspica GRAS transcription factor, enhances plant growth and salt stress tolerance in transgenic Arabidopsis[J]. Plant Physiology and Biochemistry, 2020, 151: 243−254. doi: 10.1016/j.plaphy.2020.03.020
-
期刊类型引用(3)
1. 饶丹丹,韩豫,吴二焕,陈彧. 不同光强下琼岛杨幼苗生长和光合特性. 山西农业大学学报(自然科学版). 2024(01): 61-69 .  百度学术
百度学术
2. 韩霜,徐浩,余静雅,韩赟,张发起. 藏茵陈基源植物皱边喉毛花的全长转录组信息分析. 广西植物. 2023(07): 1335-1346 .  百度学术
百度学术
3. 郝豆豆,张勇群,施静,拉多,雷鸣. 报春花科3种植物对青藏高原适应性进化的转录组学研究. 西部林业科学. 2022(04): 141-147 .  百度学术
百度学术
其他类型引用(2)



 下载:
下载: