Cloning and functional analysis of heat shock protein SpHSP70-3 gene from Sorbus pohuashanensis
-
摘要:目的 探讨热激蛋白(HSP)在花楸树响应高温胁迫过程中的作用,以期为花楸树引种低海拔地区提供理论基础。方法 以2 ~ 4年生花楸树实生苗为研究对象,进行了花楸树SpHSP70-3基因的克隆、系统进化分析、组织特异性表达模式以及响应高温胁迫的表达机制研究,并利用农杆菌介导法转化拟南芥,对SpHSP70-3基因在高温胁迫下的响应进行了异源验证。结果 SpHSP70-3基因开放阅读框全长为2 088 bp,编码695个氨基酸;SpHSP70-3蛋白与蔷薇科梨属的白梨PbHSP70同源性最高。内源性表达分析显示:SpHSP70-3基因在叶片中表达量最高,在花蕾、初花、盛花时表达量偏低;42 ℃处理花楸树后,发现前6 h SpHSP70-3基因表达量没有显著变化,12 h时表达量增至对照组的12倍,24 h时表达倍数最高,为对照组的19倍。对3个转SpHSP70-3基因拟南芥纯合株系(OE1、OE2、OE3)和野生型拟南芥(WT)进行45 ℃高温处理后,OE1、OE2、OE3中的丙二醛(MDA)含量均高于WT,且过氧化氢酶(CAT)活性和过氧化物酶(POD)活性结果均低于WT。此外,SpHSP70-3在转基因株系中的表达量随着处理时间的增加而上升,并且其抑制了正调节因子AtHSP70、AtHSP18.2、AtHsfA1D和AtHsfA1A的表达,同时诱导了负调节因子AtHsfB2B的上调表达。结论 SpHSP70-3在花楸树响应高温胁迫过程中起负调控作用,初步推测SpHSP70-3是花楸树引种低海拔地区响应高温响胁迫机制中的负调控因子。Abstract:Objective This paper aims to explore the function of heat shock protein (HSP) in Sorbus pohuashanensis in response to high temperature stress, and to provide theoretical basis for the introduction of species in low altitude areas.Method Taking 2−4 years old seedlings of Sorbus pohuashanensis as the research object, the cloning, phylogenetic analysis, tissue-specific expression pattern and expression mechanism in response to high temperature stress of Sorbus pohuashanensis were studied, and the response function of transgenic Arabidopsis thaliana to high temperature stress was verified by agrobacterium-mediated transformation.Result The full-length open reading frame of SpHSP70-3 gene was 2 088 bp, encoding 695 amino acids. SpHSP70-3 protein had the highest homology with Pyrus bretschneideri PbHSP70 in Rosaceae. Endogenous expression analysis showed that the expression of SpHSP70-3 gene was the highest in leaves, but the expression was low in bud, first flower and full flower. The expression level did not change significantly 6 h before treatment at 42 ℃, but increased to 12 times of the control group at 12 h, and reached the highest level at 24 h, but only 19 times of the control group. After three homozygous arabidopsis lines of OE1, OE2 and OE3 with SpHSP70-3 gene and WT arabidopsis were treated at 45 ℃, the MDA contents in OE1, OE2 and OE3 were all higher than WT, and the CAT enzyme activity and POD enzyme activity were lower than WT. In addition, the expression of SpHSP70-3 in transgenic lines increased with the increase of treatment time, and inhibited the expression of positive regulatory factors AtHSP70, AtHSP18.2, AtHsfA1D and AtHsfA1A, while induced the up-regulation of negative regulatory factor AtHsfB2B.Conclusion SpHSP70-3 plays a negative regulatory role in the response of Sorbus pohuashanensis to high temperature stress, and preliminarily speculates that SpHSP70-3 is a negative regulatory factor in the response mechanism of Sorbus pohuashanensis to high temperature stress at low altitude.
-
热激蛋白(heat shock proteins,HSPs)是指在遭受不利环境等非生物胁迫时,被诱导激活并大量积累的一类蛋白质,其普遍存在于整个生物界,具有高度保守性[1]。在胁迫条件下,HSP70能协助异常蛋白质的重新折叠,恢复正常的蛋白质构象并维持细胞稳态,对保护植物在抗逆过程中免受伤害起着非常重要的作用[2]。研究发现:HSP70过量表达不但能够使植株的耐热性能(或耐高温性能)得到有效地提高,而且还能响应干旱、低温、盐渍化、重金属、激素等不良环境[3-9]。比如玉米(Zea mays)在42 ℃热激2 h后和低温处理4 h后,ZmHSP70诱导合成,而且幼苗的抗寒能力有所增强,有助于幼苗的生长[10]。水稻(Oryza sativa)OsHSP70基因在盐和干旱胁迫下,表达量也都有明显的上调表达[11]。而缺乏AtHSP70-15的拟南芥(Arabidopsis thaliana)植株在热处理后,死亡率极大地增加,这表明AtHSP70-15在拟南芥正常生长期间对热应激发挥了重要作用[12]。然而并不是所有的HSP70基因在高温胁迫条件下都能够上调表达从而提高植物抵御高温伤害的能力,Batcho等[13]对龙舌兰(Agave sisalana)热激处理后发现:有3个诱导型HSP70的相对表达量均低于对照组,推测这与应激耐受性的降低密切相关;Vitale等[14]对已经被转入酵母BiP基因的烟草进行高温胁迫后发现:在高温处理下的转基因烟草中的HSP70和BiP蛋白的表达水平都有一定幅度的上升,但是转基因型的抗高温能力并没有很明显的提高。这些研究表明有些HSP70s在植物应答高温胁迫时起负向作用。
花楸树(Sorbus pohuashanensis)是蔷薇科(Rosaceae)花楸属(Sorbus)落叶小乔木,是我国重要的乡土景观树种,具有很高的观赏价值[15-16]、药用价值及生态价值[17-18],且抗寒性强,非常适用于北方园林绿化中[19]。但其大多自然生长在海拔800 ~ 2 000 m的阴坡、山谷、杂木林内等生境中,喜凉爽的环境,这种独特的生存环境很大程度地影响了花楸树在低海拔地区对夏季高温环境的适应性,尤其在夏季高温的环境下很容易发生叶片“日灼”[20]。目前对花楸树的研究集中在种群遗传、繁殖方法(播种、扦插、组织培养)、耐荫性及引种驯化等方面[21-29],关于花楸树对高温胁迫的响应机制方面仅存在生理生化层面[30]、小热激蛋白[31-32]及热激蛋白70-1[33]的研究。最近裴鑫等[34]采用转录组技术探索了花楸树响应高温胁迫的分子调控网络,并鉴定了一些与高温胁迫响应相关的基因,主要涉及信号转导、热激转录因子−热激蛋白以及活性氧(ROS)等途径。在前人研究基础上,本研究克隆了SpHSP70-3基因并转化拟南芥进行了功能分析,以期为今后花楸树高温胁迫下的分子应答机制的研究奠定基础,为花楸树引种驯化及耐热品种选育提供依据。
1. 材料与方法
1.1 植物材料
花楸树叶片材料来自于北京昌平区北京农学院花楸种质资源圃。野生型拟南芥(Col)为本实验室保存。
1.2 试验方法
1.2.1 总RNA、gDNA的提取及第一链cDNA的合成
参照EASYspin Plus Complex Plant RNA Kit(Aidlab,RN53)提取花楸树叶片的RNA后,根据EasyScript One-Step gDNA Removal and cDNA Synthesis SuperMix(TransGen,AT311)说明进行反转录,每20 µL反转录体系中加入1 µg RNA,合成第一链cDNA。参照Plant Genomic DNA Kit(康为世纪,CW0531s)提取花楸树gDNA。
1.2.2 基因克隆及序列分析
由花楸树叶片转录组数据分析预测,获得1条HSP70家族相关基因序列,命名为SpHSP70-3。根据基因序列设计克隆引物(表1),分别以花楸树cDNA和gDNA为模板进行PCR扩增,反应体系:模板1 μL、上下游引物各0.4 μL、2 × Master Mix 10 μL、ddH2O 8.2 μL。扩增程序:95 ℃ 3 min,95 ℃ 30 s,58 ℃ 30 s,72 ℃ 100 s,72 ℃ 5 min,4 ℃ 终止保温,33个循环。PCR产物经1.0%琼脂糖凝胶电泳检验后回收纯化目的条带,并连接到pEASY®-T1克隆载体(TransGen)进行测序,采用DNAMAN 7.0软件对测序结果进行ORF分析,在NCBI数据库(https://www.ncbi.nlm.gov/Stucture/cdd/wrpsb.cgi)对蛋白的氨基酸序列进行结构域的分析。
表 1 引物序列Table 1. Primer sequence引物名称 Primer name 序列(5′—3′) Sequence ( 5′−3′ ) 引物名称 Primer name 序列(5′—3′) Sequence ( 5−3′ ) SpHSP70-3-F ATGGCTTCCGCGCAAA SpHSP70-3-R CTCGCTCACCTGCTGTCG SpHSP70-3-TF CACCATGGCTTCCGCGCAAA SpHSP70-3-TR CTCGCTCACCTGCTGTCG Spβ-actin-QF TGGATGGCTGGAAGAGGA Spβ-actin-QR GAGCGGGAAATTGTGAGG SpHSP70-3-QF TCTCTTCTCCTTGTCCTCCTG SpHSP70-3-QR TTCTATCCGTCGCTGCTGT AtHSP70-QF ACTTGCTTATGAGTCTGAGGGTA AtHSP70-QR GCCTTGATAGGTGCTGATAGA AtHSP90-QF GGGGATTTGAACCTTATTGGA AtHSP90-QR CTGGCTGTCATCATTGTGCTT AtHSP18.2-QF CCGTTCTCGCAAGACTTATGG AtHSP18.2-QR CGGCGTTTCCTTCCAATCCAC AtHsfA1D-QF AGAAGCAACCGAGAACTGTAT AtHsfA1D-QR AGTAATGGACTAGAACCTCCC AtHsfA1A-QF TGGAGTCCGACGAACAATAGC AtHsfA1A-QR GGCGAACAAAGCTGGAGAAAT AtHsfB2B-QF AGTAGTGGATGTGGTGCTGGTG AtHsfB2B-QR CGAGATCAATTCGTCGTAAACC 1.2.3 SpHSP70-3蛋白系统进化分析
在NCBI网站上用BLAST工具下载同源蛋白序列后,使用DNAMAN进行多序列比对。采用N-J法使用Mega 7软件构建系统发育树,并采用bootstraps法重复1 000次。
1.2.4 SpHSP70-3基因的内源性表达
于2017年4—10月期间,在北京农学院花楸树种质资源圃中选取4年生嫁接苗3棵,采集其幼叶、成叶、花蕾、初花(花瓣尚未完全展开)、盛花(花瓣完全打开,雄雌蕊成熟)、末花(花瓣褐化,萎蔫)、果实、茎和根,进行花楸树组织特异性表达。选取长势一致的2年生花楸树实生苗放在温度为42 ℃、光照为120 μmol/(m2·s)的培养箱中分别进行0、0.25、1、2、6、12、24 h的高温胁迫处理,处理结束后取中上部叶片,3次生物学重复。分别提取上述不同组织和不同胁迫时间点花楸叶片的总RNA,反转录成cDNA,并设计SpHSP70-3荧光定量引物(表1)。反应体系参照1.2.2,反应程序为:94 ℃预变性30 s;94 ℃变性5 s,50 ~ 60 ℃退火15 s,72 ℃延伸10 s,40个循环;55 ℃,30 s,81个循环。3次技术重复。组织特异性定量结果用2−ΔΔCT方法分析,42 ℃定量结果用2-ΔΔCT方法分析。
1.2.5 转SpHSP70-3基因纯和拟南芥的获得
利用InvitrogenTM GatewayTM重组克隆技术,构建超表达载体p35S:SpHSP70-3,并将其转化至GV3101 + MPK90农杆菌中,利用花絮浸染法转化拟南芥。收集侵染后的转SpHSP70-3基因拟南芥(T0代)种子,首先使用7%次氯酸钠溶液消毒10 min,再用75%的乙醇洗30 s除去残留次氯酸钠,最后用无菌水洗涤3 ~ 4次,每次3 min。消毒后,将种子播种在含0.1%草铵膦的1/2MS培养基上,4 ℃春化3 d后放置人工气候箱培养,培养条件为:相对湿度60% ~ 70%,16 h光培养(23 ℃),8 h暗培养(20 ℃),光强120 μmol/(m2·s)。培养一周后筛选出生长正常的幼苗转移至基质中继续培养收种。连续筛选3代后获得3个转SpHSP70-3基因纯合拟南芥株系OE1、OE2和OE3,并以克隆引物(表1)进行PCR验证。
1.2.6 转SpHSP70-3基因拟南芥高温胁迫下生理指标的测定
将在1/2MS培养基中正常生长10 d的野生型(WT)和转SpHSP70-3拟南芥幼苗转移到45 ℃的培养箱中,分别热激0、2、4、6和8 h,然后恢复正常培养5 d,观察表型变化。收集叶片后,使用丙二醛(malondialdehyde,MDA)含量(Solarbio,BC0020)、过氧化氢酶(catalase,CAT)活性(Solarbio,BC0200)及过氧化物酶(peroxidase,POD)活性(Solarbio,BC0090)检测试剂盒进行相关指标测定。每个基因型分别50株苗,3次重复。
1.2.7 转SpHSP70-3基因拟南芥高温胁迫下的基因表达
提取高温处理下的各基因型拟南芥叶片RNA后,并反转录为cDNA,以Atactin-QF、Atactin-QR为内参引物[35],进行SpHSP70-3基因在高温胁迫下的时间差异表达分析。PCR反应体系参照1.2.2。反应程序为94 ℃ 30 s、94 ℃ 5 s、59 ℃ 15 s、72 ℃ 10 s,循环数40。结果采用2-△△CT法计算,每个样品进行3个技术重复。
在拟南芥数据库TAIR(https://www.arabidopsis.org/)中选取热激网络通路中的6个相关基因并设计引物(表1),分别为AtHSP70(NM-117767)、AtHSP90(NM-118552)、AtHSP18.2(NM-125346)、AtHsfA1D(NM-102966)、AtHsfA1A(NM-117884)和AtHsfB2B(NM-117235),对45 ℃热激4 h后的WT和转SpHSP70-3基因拟南芥进行实时荧光定量分析。
采用软件SPSS 17.0独立样本T检验和单因素方差分析对WT和转SpHSP70-3基因拟南芥的生理指标和基因表达的数据进行处理。
2. 结果与分析
2.1 花楸树SpHSP70-3基因序列分析
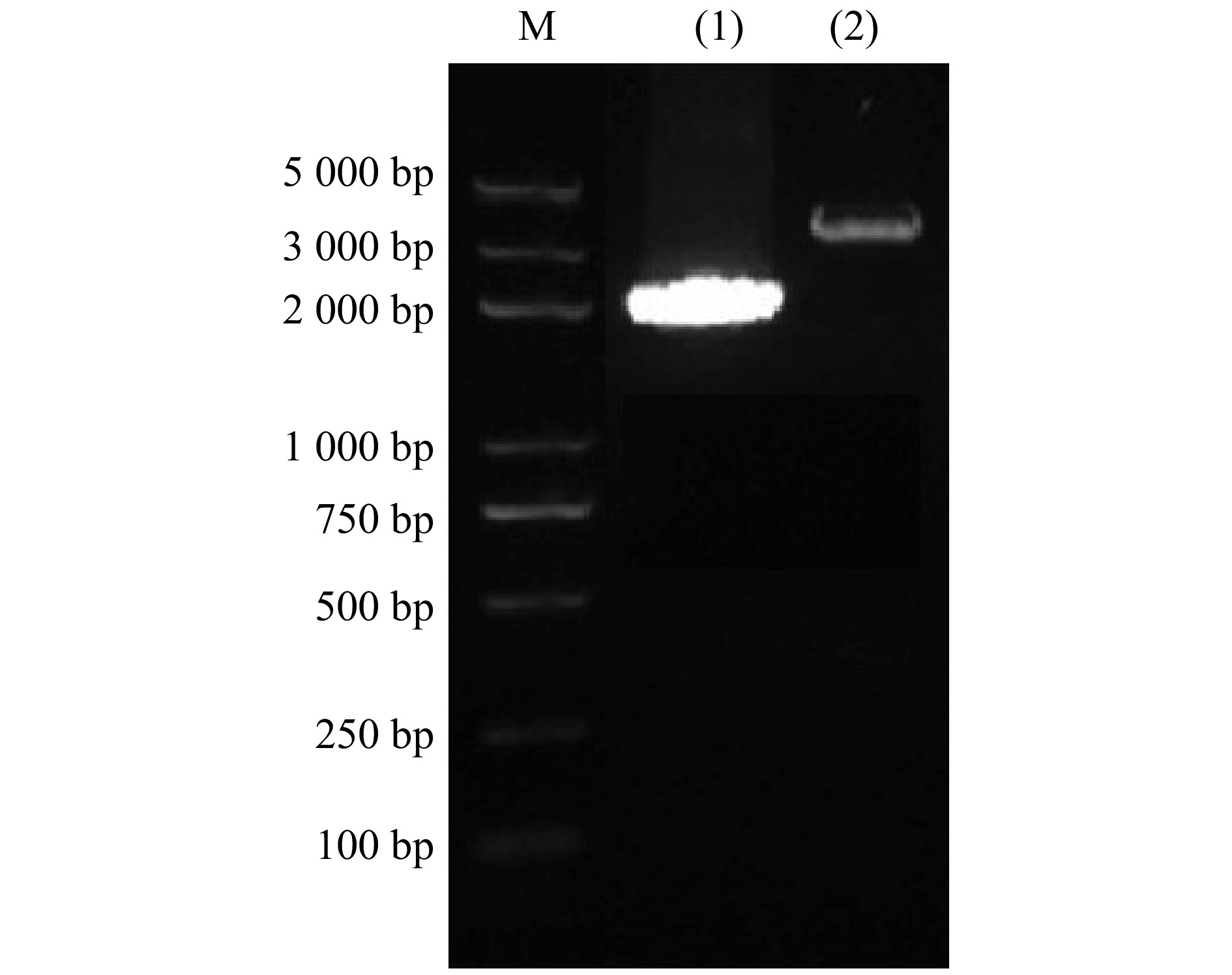
根据花楸树叶片转录组数据,经NCBI网站BLAST比对筛选出1条具有全长序列的HSP70家族基因,命名为SpHSP70-3。以花楸树叶片cDNA为模板进行PCR扩增,得到长度为2 000 bp左右的片段(图1),后将其连接到pEASY-T1载体上测序,测序结果与转录组测序结果一致,确定扩增得到的序列为花楸树SpHSP70-3基因的ORF序列,该序列长度为2 088 bp,编码695个氨基酸(图2)。SpHSP70-3蛋白氨基酸序列包含3种保守基序,分别为17个核苷酸结合位点、19个核苷酸互换因子互作位点和11个底物结构域(图2)。
2.2 SpHSP70-3蛋白系统进化分析
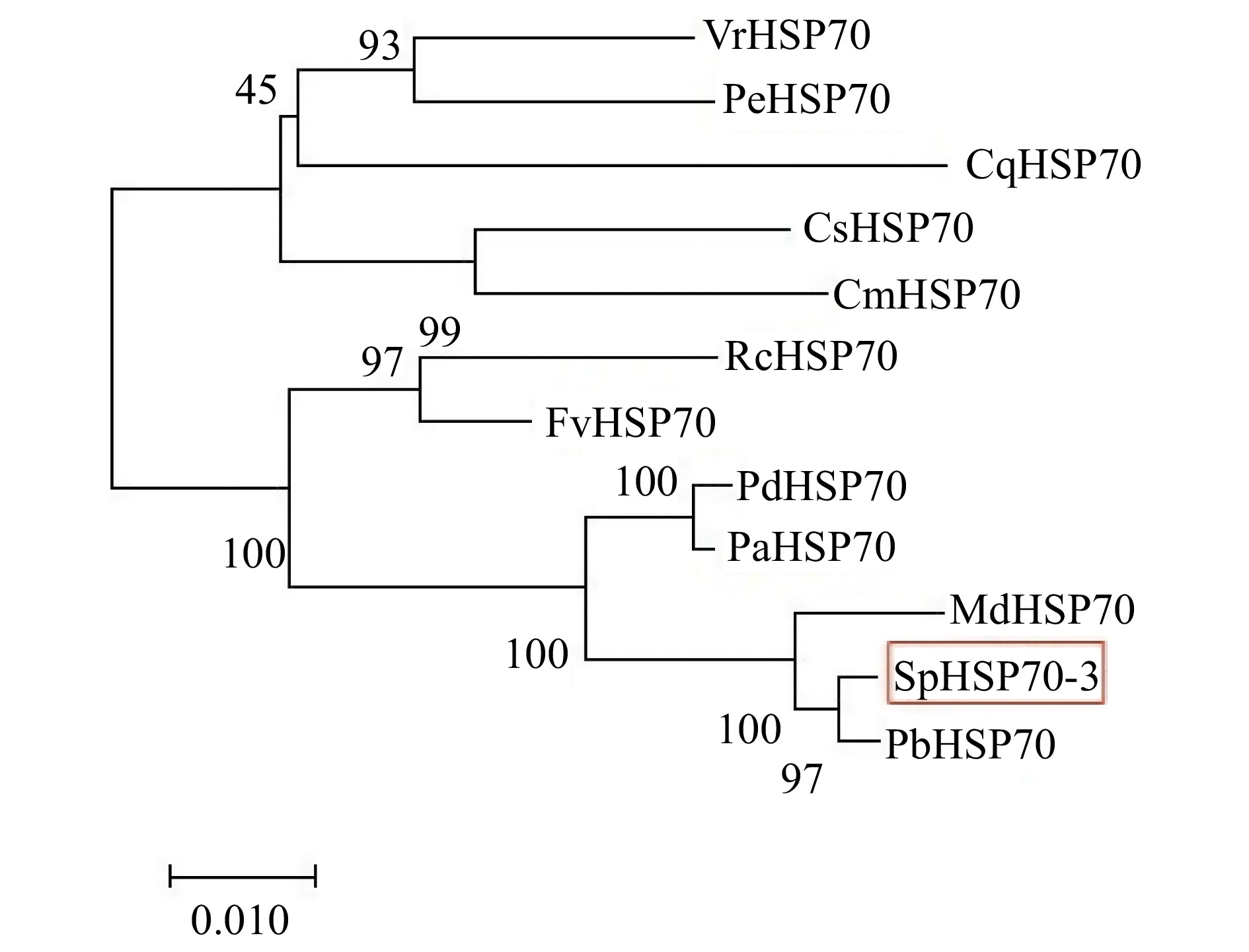
SpHSP70-3蛋白与其他植物的同源序列比对结果显示:花楸树SpHSP70-3与白梨(Pyrus bretschneideri)、苹果(Malus domestica)的同源性最高,分别为99.28%和98.13%,与蔷薇科其他属的植物如甜樱桃(Prunus avium)、扁桃(Prunus dulcis)、草莓(Fragaria vesca subsp. vesca)、月季(Rosa chinensis)等同源性也较高,均达到了90%以上,而与其他科的植物同源性则较低,分别为80%左右(图3)。保守结构域预测显示:DNA结合域(NBD)位于56 ~ 430氨基酸处,C端底物结合域(SBD)位于555 ~ 653处(图3)。
![]() 图 3 SpHSP70-3蛋白与其他植物HSP70的同源序列比对Cm. 南瓜 Cucurbita moschata (XP_022930091.1);Cq. 藜麦 Chenopodium quinoa (XP_021743068.1);Cs. 黄瓜 Cucumis sativus (NP_001295865.1);Fv. 草莓 Fragaria vesca subsp. vesca (XP_004300629.1);Md. 苹果 Malus domestica (XP_028963255.1);Pb. 白梨 Pyrus bretschneideri (XP_009353782.1);Pa. 甜樱桃 Prunus avium (XP_021815855.1);Pd. 扁桃 Prunus dulcis (XP_034211692.1);Rc. 月季 Rosa chinensis (XP_024170474.1);Pe. 胡杨 Populus euphratica (XP_011016196.1);Vr. 葡萄 Vitis riparia (XP_034673011.1);NBD. DNA结合域 Nucleotide binding domain;SBD. 底物结合域 Substrate binding domain.Figure 3. Homologous alignment of SpHSP70-3 protein with HSP70 protein in other plants
图 3 SpHSP70-3蛋白与其他植物HSP70的同源序列比对Cm. 南瓜 Cucurbita moschata (XP_022930091.1);Cq. 藜麦 Chenopodium quinoa (XP_021743068.1);Cs. 黄瓜 Cucumis sativus (NP_001295865.1);Fv. 草莓 Fragaria vesca subsp. vesca (XP_004300629.1);Md. 苹果 Malus domestica (XP_028963255.1);Pb. 白梨 Pyrus bretschneideri (XP_009353782.1);Pa. 甜樱桃 Prunus avium (XP_021815855.1);Pd. 扁桃 Prunus dulcis (XP_034211692.1);Rc. 月季 Rosa chinensis (XP_024170474.1);Pe. 胡杨 Populus euphratica (XP_011016196.1);Vr. 葡萄 Vitis riparia (XP_034673011.1);NBD. DNA结合域 Nucleotide binding domain;SBD. 底物结合域 Substrate binding domain.Figure 3. Homologous alignment of SpHSP70-3 protein with HSP70 protein in other plantsSpHSP70-3蛋白与上述11种植物的系统进化树发生结果显示:花楸树SpHSP70-3蛋白首先与蔷薇科同源性最高的白梨和苹果聚合,接着再与蔷薇科其他属的植物聚合,最后与葫芦科(Cucurbitaceae)、杨柳科(Salicaceae)、葡萄科(Vitaceae)、藜科(Chenopodiaceae)等植物聚合(图4),表明花楸树SpHSP70-3蛋白与白梨和苹果的亲缘关系最近,与其他科植物的亲缘关系较远。这一结果与序列比对结果一致。
2.3 SpHSP70-3基因的内源性表达
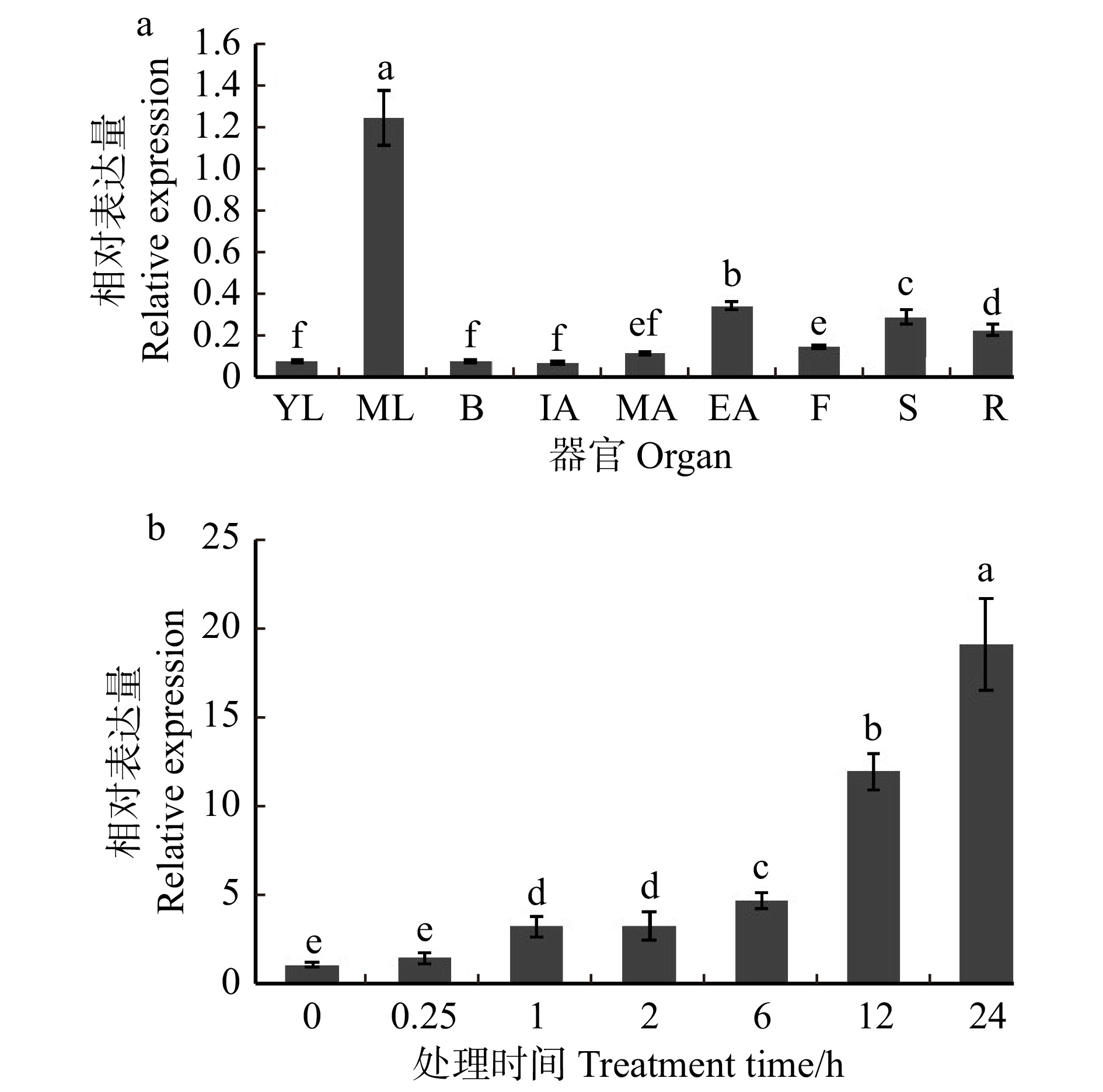
为探究SpHSP70-3基因在不同器官的不同发育时期的表达情况,取其幼叶、成叶、花蕾、初花、盛花、末花、果实、茎、根等材料进行SpHSP70-3基因的qRT-PCR检测。结果如图5所示:SpHSP70-3在成叶中大量表达,于末花、茎、根中表达量较低,在花蕾、初花、盛花时表达量最低(图5a)。在SpHSP70-3响应热胁迫的瞬时表达模式中,发现SpHSP70-3表达量在24 h之内逐渐上升,在前6 h处理中并无显著倍数变化,12 h时表达量增至CK的12倍,24 h时表达倍数最高,为CK的19倍(图5b)。
![]() 图 5 花楸树SpHSP70-3基因的内源性表达分析a. SpHSP70-3基因组织特异性表达;b. 42 ℃高温胁迫下SpHSP70-3基因表达分析。YL. 幼叶;ML. 成叶;B. 花蕾;IA. 初花;MA. 盛花;EA. 末花;F. 果;S. 茎;R. 根。不同小写字母表示差异显著,P < 0.05。下同。a, tissue specific expression of SpHSP70-3; b, relative expression of SpHSP70-3 at 42 ℃ treatment . YL, younger leaf; ML, mature leaf; B, bud; IA, initial anthesis; MA, mature anthesis; EA, end anthesis; F, fruit; S, stem; R, root. Different letters represent significant differences at P < 0.05 level. The same below.Figure 5. Endogenous expression analysis of SpHSP70-3 gene inSorbus pohuashanensis
图 5 花楸树SpHSP70-3基因的内源性表达分析a. SpHSP70-3基因组织特异性表达;b. 42 ℃高温胁迫下SpHSP70-3基因表达分析。YL. 幼叶;ML. 成叶;B. 花蕾;IA. 初花;MA. 盛花;EA. 末花;F. 果;S. 茎;R. 根。不同小写字母表示差异显著,P < 0.05。下同。a, tissue specific expression of SpHSP70-3; b, relative expression of SpHSP70-3 at 42 ℃ treatment . YL, younger leaf; ML, mature leaf; B, bud; IA, initial anthesis; MA, mature anthesis; EA, end anthesis; F, fruit; S, stem; R, root. Different letters represent significant differences at P < 0.05 level. The same below.Figure 5. Endogenous expression analysis of SpHSP70-3 gene inSorbus pohuashanensis2.4 转SpHSP70-3基因拟南芥高温胁迫下基因表达量的分析
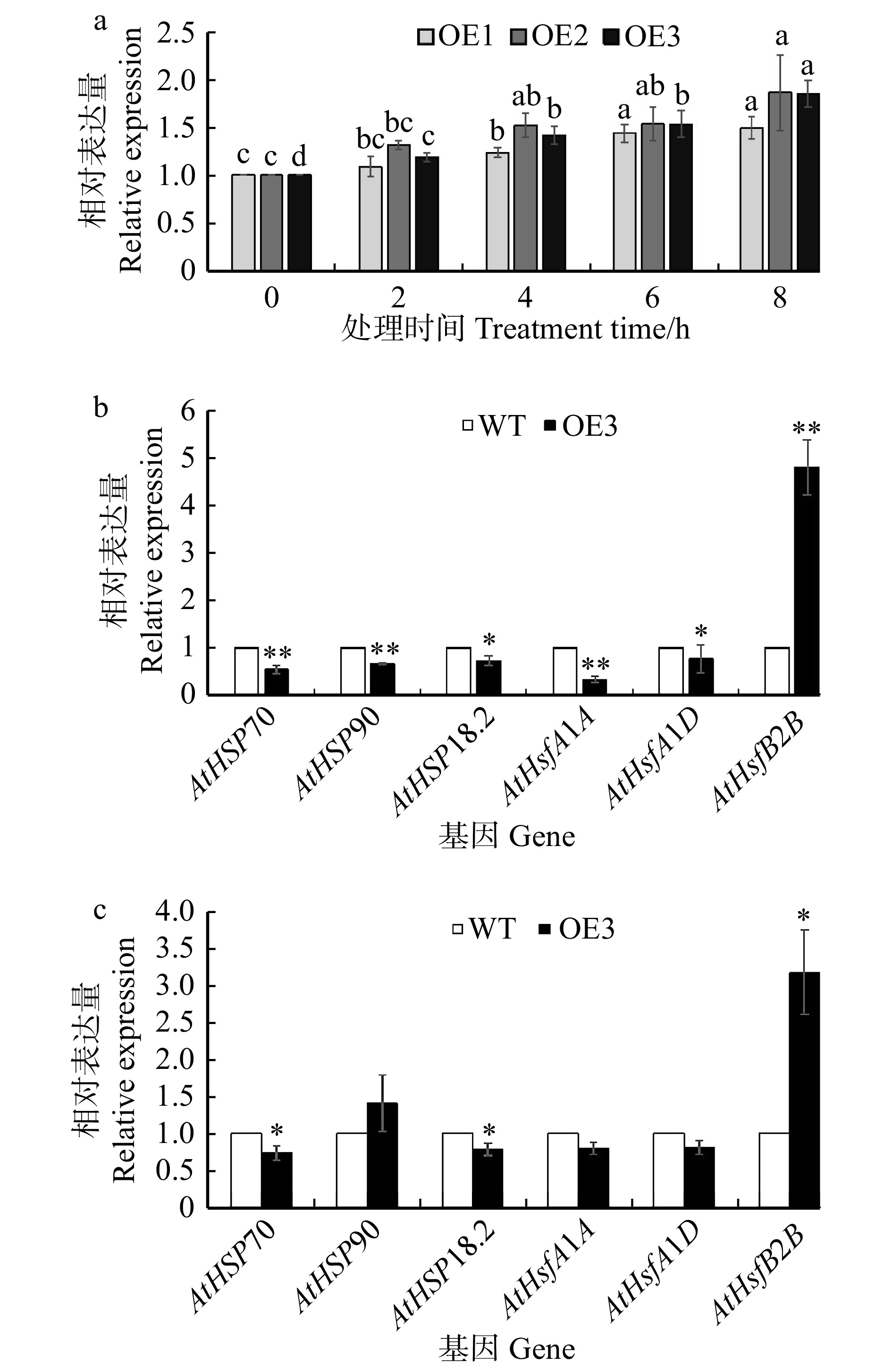
经3代筛选得到了3个纯合转基因株系OE1、OE2和OE3。分别对OE1、OE2、OE3进行SpHSP70-3基因在高温胁迫下的表达分析,结果显示:转SpHSP70-3基因拟南芥在45 ℃热激2、4、6、8 h后,SpHSP70-3均上调表达(图6a),与其在花楸树受到高温胁迫时的表达趋势一致。此外,还选取了拟南芥热激网络通路中的6个相关基因:AtHSP70、AtHSP90、AtHSP18.2、AtHsfA1D、AtHsfA1A和AtHsfB2B,对SpHSP70-3的调控机理进行了探究。结果表明:在正常环境中,转SpHSP70-3基因拟南芥的AtHSP70、AtHSP90、AtHSP18.2、AtHsfA1D和AtHsfA1A表达量均显著低于WT中的表达量,而AtHsfB2B表达量却极显著高于WT(图6b);45 ℃热激处理4 h后,发现转SpHSP70-3基因拟南芥的AtHSP70、AtHSP18.2、AtHsfA1D和AtHsfA1A基因表达量均低于WT,而AtHsfB2B表达量显著高于WT(图6c)。
![]() 图 6 45 ℃胁迫下转SpHSP70-3基因拟南芥基因表达分析a. 转基因拟南芥OE1、OE2、OE3在45 ℃下的SpHSP70-3基因表达;b. 正常条件下株系OE3和WT中基因表达分析;c. 45 ℃处理下株系OE3和WT中基因表达分析。*代表P < 0.05平上的显著差异,**代表在P < 0.01水平上的显著差异,下同。a, expression analysis of SpHSP70-3 gene in transgenic Arabidopsis thaliana under 45 ℃ stress; b, analysis of target gene expression in OE3 and WT under normal conditions; c, analysis of target gene expression in OE3 and WT under 45 ℃ stress. * represents significance at P < 0.05 level, ** represents significance at P < 0.01 level. The same below.Figure 6. Expression analysis of genes in transgenic Arabidopsis thaliana under 45 ℃ stress
图 6 45 ℃胁迫下转SpHSP70-3基因拟南芥基因表达分析a. 转基因拟南芥OE1、OE2、OE3在45 ℃下的SpHSP70-3基因表达;b. 正常条件下株系OE3和WT中基因表达分析;c. 45 ℃处理下株系OE3和WT中基因表达分析。*代表P < 0.05平上的显著差异,**代表在P < 0.01水平上的显著差异,下同。a, expression analysis of SpHSP70-3 gene in transgenic Arabidopsis thaliana under 45 ℃ stress; b, analysis of target gene expression in OE3 and WT under normal conditions; c, analysis of target gene expression in OE3 and WT under 45 ℃ stress. * represents significance at P < 0.05 level, ** represents significance at P < 0.01 level. The same below.Figure 6. Expression analysis of genes in transgenic Arabidopsis thaliana under 45 ℃ stress2.5 转SpHSP70-3基因拟南芥高温胁迫下生理指标的分析
以10 d龄的T3代拟南芥幼苗进行45 ℃胁迫后转到正常条件下继续培养5 d,结果发现:在2 h和4 h时,WT明显比转SpHSP70-3基因拟南芥长势好,黄化苗较少(图7a)。对在45 ℃高温下生长0 h和4 h的WT和3个株系OE1、OE2、OE3拟南芥进行MDA、CAT、POD等生理指标测定,结果显示:0 h时,MDA含量在WT和OE1、OE2、OE3之间并没有显著差异;但在4 h时,MDA含量在WT和OE1、OE2、OE3中分别有所提高,但转SpHSP70-3基因株系中的MDA含量都高于WT,且OE2、OE3显著或极显著高于WT(图7b)。 酶活性结果显示:转SpHSP70-3基因株系中的CAT和POD酶活性都低于WT,其中OE3显著低于WT(图7c、7d),说明转SpHSP70-3基因拟南芥受到高温的伤害程度高于WT,且抗氧化酶活性受到了抑制。
![]() 图 7 45 ℃胁迫下转SpHSP70-3基因和野生型拟南芥的表型及生理指标分析a. 转SpHSP70-3基因拟南芥OE1、OE2、OE3株系在45 ℃下的胁迫表型;b. MDA含量分析;c. CAT酶活性分析;d. POD酶活性分析。a, phenotype of transgenic Arabidopsis thaliana with SpHSP70-3 gene under 45 ℃ stress; b, analysis of MDA content; c, analysis of CAT activity; d, analysis of POD activity.Figure 7. Phenotypic and physiological indexes analysis of transgenic SpHSP70-3 gene and wild Arabidopsis thaliana under 45 ℃ stress
图 7 45 ℃胁迫下转SpHSP70-3基因和野生型拟南芥的表型及生理指标分析a. 转SpHSP70-3基因拟南芥OE1、OE2、OE3株系在45 ℃下的胁迫表型;b. MDA含量分析;c. CAT酶活性分析;d. POD酶活性分析。a, phenotype of transgenic Arabidopsis thaliana with SpHSP70-3 gene under 45 ℃ stress; b, analysis of MDA content; c, analysis of CAT activity; d, analysis of POD activity.Figure 7. Phenotypic and physiological indexes analysis of transgenic SpHSP70-3 gene and wild Arabidopsis thaliana under 45 ℃ stress3. 讨论与结论
研究[2]报道,HSP70蛋白在植物响应逆境胁迫的过程中具有非常重要的作用,并作为分子伴侣协助异常蛋白质的重新折叠,恢复正常的蛋白质构象并维持细胞稳态,对生物体正常机能的维护有重大意义。本研究从花楸树中克隆1个SpHSP70-3基因的ORF,全长为2 088 bp,编码695个氨基酸。将SpHSP70-3与其他11个物种的HSP70蛋白进行同源序列比对,发现花楸树SpHSP70-3与蔷薇科的白梨、苹果、甜樱桃、扁桃、草莓及月季植物的SpHSP70同源性较高。同时发现较为保守的NBD和SBD结构域集中出现在N端,C端可变性较大,而HSP70s通常是由持续的NBD和SBD以及可变的C末端组成,灵活的C末端有利于底物与SBD结合[36]。对SpHSP70-3在花楸树不同组织中和不同高温胁迫时间点进行表达分析,发现SpHSP70-3在根、茎、叶及花组织中均有表达,在成叶中表达量最高,表明其可能在叶片发育过程中发挥了重要作用。在拟南芥中,与SpHSP70-3编码的氨基酸序列同源性最高的热激蛋白70为AtHSC70-7,该基因是定位于叶绿体的基因[37]。由此推测SpHSP70-3可能在叶绿体中表达,因此SpHSP70-3在成熟叶片中相较其他器官大量表达。花楸树在42 ℃的胁迫下,SpHSP70-3的表达量随着胁迫时间的延长逐渐上调,说明SpHSP70-3可以响应高温胁迫。这与之前热激蛋白能够响应高温胁迫的研究结果一致,如张泽等[32]对花楸树进行高温胁迫处理后,发现叶片中SpHSP23.8基因的相对表达量显著升高,且在胁迫2 h时达到峰值,说明SpHSP23.8能够积极响应高温胁迫。刘聪聪等[33]发现花楸树叶片在热处理24 h内,SpHSP70-1均有几十倍至几百倍的高表达变化,在热处理1 h时,表达量达到最高,说明SpHSP70-1可以响应高温胁迫。
为了进一步研究SpHSP70-3的耐高温功能,对3个转SpHSP70-3基因拟南芥株系和WT在高温(45 ℃)胁迫下进行生理生化指标测定和基因表达分析。结果显示:转SpHSP70-3基因拟南芥中抗氧化酶活性受到了抑制,不能有效地发挥其清除活性氧的功能,从而导致了细胞内过氧化产物MDA的积累,使转SpHSP70-3基因拟南芥受高温的伤害程度高于WT。有研究[38]表明:野生型和DBB1ɑ拟南芥突变体经高温处理后,DBB1ɑ突变体中AtHSP70、AtHSP101表达量均低于野生型,而且其相对电导率和MDA含量也都高于WT。徐红云等[39]发现在渗透胁迫下AtSCL4对AtSOD1和PER4的表达具有负调控作用,进而影响SOD和POD的合成。因此,我们推测SpHSP70-3基因可能通过调控POD、SOD酶等相关基因的表达抑制了POD和SOD的活性,从而使ROS在逆境中积累,进而降低花楸树的耐高温能力。
对转SpHSP70-3基因拟南芥株系的高温(45 ℃)胁迫结果显示:转SpHSP70-3基因拟南芥中SpHSP70-3基因的相对表达量的变化趋势与花楸树在高温胁迫时的表达趋势一致,进一步表明SpHSP70-3基因参与了花楸树对高温胁迫的响应。而SpHSP70-3的过表达却抑制了正调节因子AtHSP70、AtHSP18.2、AtHsfA1D和AtHsfA1A的表达,同时诱导了负调节因子AtHsfB2B的上调表达,导致转基因拟南芥株系的耐热性降低,这一结果与Liu等[40]对拟南芥AtHSFA1和Miho等[41]对拟南芥HsfB1、HsfB2b的功能研究结果一致,因此推测SpHSP70-3是高温响应机制中的负调控因子。有研究表明:过表达AtHSC70-1拟南芥通过负调节转录因子HsfA1d、HsfA1e、HsfA2的表达,进一步抑制AtHSP101的活性,从而负调控拟南芥对高温的耐受性[42]。姚紫薇等[43]发现受高温诱导的HSFB2b通过识别热激元件(heat shock element,HSE),负调控茉莉酸降解相关基因ST2A的表达,从而负调控植物热形态建成。因此推测SpHSP70-3诱导AtHsfB2B大量表达,AtHsfB2B与正调节因子HSE元件结合,从而抑制热响应相关基因的表达,进而负调控花楸树的热胁迫响应。
综上所述,热激蛋白网络的快速响应不一定能够提高植物对于逆境的抵抗力,SpHSP70-3基因参与花楸树对高温胁迫的响应,起负调控作用。本研究为今后花楸树高温胁迫下的分子应答机制的研究奠定了基础,为花楸树引种驯化提供了依据。
-
图 3 SpHSP70-3蛋白与其他植物HSP70的同源序列比对
Cm. 南瓜 Cucurbita moschata (XP_022930091.1);Cq. 藜麦 Chenopodium quinoa (XP_021743068.1);Cs. 黄瓜 Cucumis sativus (NP_001295865.1);Fv. 草莓 Fragaria vesca subsp. vesca (XP_004300629.1);Md. 苹果 Malus domestica (XP_028963255.1);Pb. 白梨 Pyrus bretschneideri (XP_009353782.1);Pa. 甜樱桃 Prunus avium (XP_021815855.1);Pd. 扁桃 Prunus dulcis (XP_034211692.1);Rc. 月季 Rosa chinensis (XP_024170474.1);Pe. 胡杨 Populus euphratica (XP_011016196.1);Vr. 葡萄 Vitis riparia (XP_034673011.1);NBD. DNA结合域 Nucleotide binding domain;SBD. 底物结合域 Substrate binding domain.
Figure 3. Homologous alignment of SpHSP70-3 protein with HSP70 protein in other plants
图 5 花楸树SpHSP70-3基因的内源性表达分析
a. SpHSP70-3基因组织特异性表达;b. 42 ℃高温胁迫下SpHSP70-3基因表达分析。YL. 幼叶;ML. 成叶;B. 花蕾;IA. 初花;MA. 盛花;EA. 末花;F. 果;S. 茎;R. 根。不同小写字母表示差异显著,P < 0.05。下同。a, tissue specific expression of SpHSP70-3; b, relative expression of SpHSP70-3 at 42 ℃ treatment . YL, younger leaf; ML, mature leaf; B, bud; IA, initial anthesis; MA, mature anthesis; EA, end anthesis; F, fruit; S, stem; R, root. Different letters represent significant differences at P < 0.05 level. The same below.
Figure 5. Endogenous expression analysis of SpHSP70-3 gene inSorbus pohuashanensis
图 6 45 ℃胁迫下转SpHSP70-3基因拟南芥基因表达分析
a. 转基因拟南芥OE1、OE2、OE3在45 ℃下的SpHSP70-3基因表达;b. 正常条件下株系OE3和WT中基因表达分析;c. 45 ℃处理下株系OE3和WT中基因表达分析。*代表P < 0.05平上的显著差异,**代表在P < 0.01水平上的显著差异,下同。a, expression analysis of SpHSP70-3 gene in transgenic Arabidopsis thaliana under 45 ℃ stress; b, analysis of target gene expression in OE3 and WT under normal conditions; c, analysis of target gene expression in OE3 and WT under 45 ℃ stress. * represents significance at P < 0.05 level, ** represents significance at P < 0.01 level. The same below.
Figure 6. Expression analysis of genes in transgenic Arabidopsis thaliana under 45 ℃ stress
图 7 45 ℃胁迫下转SpHSP70-3基因和野生型拟南芥的表型及生理指标分析
a. 转SpHSP70-3基因拟南芥OE1、OE2、OE3株系在45 ℃下的胁迫表型;b. MDA含量分析;c. CAT酶活性分析;d. POD酶活性分析。a, phenotype of transgenic Arabidopsis thaliana with SpHSP70-3 gene under 45 ℃ stress; b, analysis of MDA content; c, analysis of CAT activity; d, analysis of POD activity.
Figure 7. Phenotypic and physiological indexes analysis of transgenic SpHSP70-3 gene and wild Arabidopsis thaliana under 45 ℃ stress
表 1 引物序列
Table 1 Primer sequence
引物名称 Primer name 序列(5′—3′) Sequence ( 5′−3′ ) 引物名称 Primer name 序列(5′—3′) Sequence ( 5−3′ ) SpHSP70-3-F ATGGCTTCCGCGCAAA SpHSP70-3-R CTCGCTCACCTGCTGTCG SpHSP70-3-TF CACCATGGCTTCCGCGCAAA SpHSP70-3-TR CTCGCTCACCTGCTGTCG Spβ-actin-QF TGGATGGCTGGAAGAGGA Spβ-actin-QR GAGCGGGAAATTGTGAGG SpHSP70-3-QF TCTCTTCTCCTTGTCCTCCTG SpHSP70-3-QR TTCTATCCGTCGCTGCTGT AtHSP70-QF ACTTGCTTATGAGTCTGAGGGTA AtHSP70-QR GCCTTGATAGGTGCTGATAGA AtHSP90-QF GGGGATTTGAACCTTATTGGA AtHSP90-QR CTGGCTGTCATCATTGTGCTT AtHSP18.2-QF CCGTTCTCGCAAGACTTATGG AtHSP18.2-QR CGGCGTTTCCTTCCAATCCAC AtHsfA1D-QF AGAAGCAACCGAGAACTGTAT AtHsfA1D-QR AGTAATGGACTAGAACCTCCC AtHsfA1A-QF TGGAGTCCGACGAACAATAGC AtHsfA1A-QR GGCGAACAAAGCTGGAGAAAT AtHsfB2B-QF AGTAGTGGATGTGGTGCTGGTG AtHsfB2B-QR CGAGATCAATTCGTCGTAAACC -
[1] Wang W, Vinocur B, Shoseyo O, et al. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response[J]. Trends in Plant Science, 2004, 9(5): 244−252. doi: 10.1016/j.tplants.2004.03.006
[2] 黄祥富, 黄上志, 傅家瑞. 植物热激蛋白的功能及其基因表达的调控[J]. 植物学通报, 1999, 16(5): 530−536. Huang X F, Huang S Z, Fu J R. The function of plant heat shock protein and its gene expression regulation[J]. Chinese Bulletin of Botany, 1999, 16(5): 530−536.
[3] Cho E K, Choi Y J. A nuclear-localized HSP70 confers thermoprotective activity and drought-stress tolerance on plants[J]. Biotechnology Letters, 2009, 31(4): 597−606. doi: 10.1007/s10529-008-9880-5
[4] Wang X Q, Yang P F, Gao Q, et al. Proteomic analysis of the response to high-salinity stress in Physcomitrella patens[J]. Planta, 2008, 228(1): 167−177. doi: 10.1007/s00425-008-0727-z
[5] Tukaj S, Bisewska J, Roeske K, et al. Time- and dose-dependent induction of HSP70 in Lemna minor exposed to different environmental stressors[J]. Bulletin of Environmental Contamination & Toxicology, 2011, 87(3): 226−230.
[6] Montero-Barrientos B, Hermosa R, Cardoza R E, et al. Transgenic expression of the Trichoderma harzianum hsp70 gene increases Arabidopsis resistance to heat and other abiotic stresses[J]. Journal of Plant Physiology, 2010, 167(8): 659−665. doi: 10.1016/j.jplph.2009.11.012
[7] Efremova S M, Margulis B A, Guzhova I V, et al. Heat shock protein Hsp70 expression and DNA damage in Baikalian sponges exposed to model pollutants and wastewater from Baikalsk Pulp and Paper Plant[J]. Aquatic Toxicology, 2002, 57(4): 267−280. doi: 10.1016/S0166-445X(01)00209-0
[8] Hu C, Lin S Y, Charng C. Recent gene duplication and subfunctionalization produced a mitochondrial GrpE, the nucleotide exchange factor of the Hsp70 complex, specialized in thermotolerance to chronic heat stress in Arabidopsis[J]. Plant Physiology, 2012, 158(2): 747−758. doi: 10.1104/pp.111.187674
[9] Frederic A, Carole L, Carsten L, et al. Virus induction of heat shock protein 70 reflects a general response to protein accumulation in the plant cytosol[J]. Plant Physiology, 2005, 138(1): 529−536. doi: 10.1104/pp.104.058958
[10] 王明强, 张道远. 植物热激蛋白70基因家族及其生物学功能研究进展[J]. 基因组学与应用生物学, 2015, 2: 421−428. doi: 10.13417/j.gab.034.000421 Wang M Q, Zhang D Y. Research progress of plant heat shock protein 70 gene family and its biological function[J]. Genomics and Applied Biology, 2015, 2: 421−428. doi: 10.13417/j.gab.034.000421
[11] Jung K H, Ko H J, Nguyen M X, et al. Genome-wide identification and analysis of early heat stress responsive genes in rice[J]. Journal of Plant Biology, 2012, 55(6): 458−468. doi: 10.1007/s12374-012-0271-z
[12] Su P H, Li H M. Arabidopsis stromal 70-kDa heat shock proteins are essential for plant development and important for thermotolerance of germinating seeds[J]. Plant Physiology, 2008, 146(3): 1231−1241. doi: 10.1104/pp.107.114496
[13] Batcho A A, Sarwar M B, Tariq L, et al. Identification and characterisation of heat shock protein gene (HSP70) family and its expression in Agave sisalana under heat stress[J]. The Journal of Horticultural Science and Biotechnology, 2020, 95(4): 1−13.
[14] Vitale A, Bielli A, Ceriotti A. The binding protein associates with monomeric phaseolin[J]. Plant Physiology, 1995, 107: 1411−1418. doi: 10.1104/pp.107.4.1411
[15] 郑勇奇, 郑健, 张川红. 花楸树: 城市绿化的新贵[J]. 中国城市林业, 2008, 6(2): 74−76. doi: 10.3969/j.issn.1672-4925.2008.02.024 Zheng Y Q, Zheng J, Zhang C H. Sorbus pohuashanens is the upstart of urban greening[J]. Journal of Chinese Urban Forestry, 2008, 6(2): 74−76. doi: 10.3969/j.issn.1672-4925.2008.02.024
[16] 张向布. 花楸树: 城市绿化的新贵[J]. 现代园艺, 2020(6): 133−134. doi: 10.3969/j.issn.1006-4958.2020.06.082 Zhang X B. Sorbus pohuashanens is the upstart of urban greening[J]. Xiandai Horticulture, 2020(6): 133−134. doi: 10.3969/j.issn.1006-4958.2020.06.082
[17] 郑健, 郑勇奇, 吴超, 等. 花楸树的地理分布及天然更新方式[J]. 林业科学, 2007, 43(12): 86−93. doi: 10.3321/j.issn:1001-7488.2007.12.015 Zheng J, Zheng Y Q, Wu C, et al. The geographical distribution and natural regeneration of Sorbus pohuashanensis[J]. Scientia Silvae Sinicae, 2007, 43(12): 86−93. doi: 10.3321/j.issn:1001-7488.2007.12.015
[18] 李莉娜, 陈兴玲. 盐碱胁迫对花楸树的影响[J]. 吉林林业科技, 2018, 47(6): 10−12. doi: 10.16115/j.cnki.issn.1005-7129.2018.06.003 Li L N, Chen X L. The effect of salt-alkali stress on Sorbus pohuashanensis[J]. Journal of Jilin Forestry Science and Technology, 2018, 47(6): 10−12. doi: 10.16115/j.cnki.issn.1005-7129.2018.06.003
[19] 郑健, 郑勇奇, 张川红, 等. 花楸树天然群体的遗传多样性研究[J]. 生物多样性, 2008, 16(6): 562−569. doi: 10.3321/j.issn:1005-0094.2008.06.006 Zheng J, Zheng Y Q, Zhang C H, et al. Study on genetic diversity of natural population of Sorbus pohuashanensis[J]. Biodiversity Science, 2008, 16(6): 562−569. doi: 10.3321/j.issn:1005-0094.2008.06.006
[20] 李涛, 许丽, 陈鹏飞, 等. 北京西山地区花楸适应性研究[J]. 林业科技通讯, 2017(10): 77−79. Li T, Xu L, Chen P F, et al. Study on the adaptability of Sorbus pohuashanensis in Xishan area of Beijing[J]. Forest Science and Technology, 2017(10): 77−79.
[21] 张振英. 遮阴和施肥对黄山花楸幼苗生长及生理的影响[D]. 南京: 南京林业大学, 2012. Zhang Z Y. Effects of shading and fertilization on the growth and physiology of Huangshan Sorbus seedlings[D]. Nanjing: Nanjing Forestry University, 2012.
[22] 刘迪. 花楸引种北京高平原和低山地区的耐热性研究[D]. 北京: 北京林业大学, 2020. Liu D. Study on the heat tolerance of Sorbus in the high plains and low mountain areas of Beijing[D]. Beijing: Beijing Forestry University, 2020.
[23] 刘迪, 孙操稳, 王立宪, 等. 不同产地花楸无性系苗期田间耐热性评价研究[J]. 北京林业大学学报, 2020, 42(4): 21−31. doi: 10.12171/j.1000-1522.20190266 Liu D, Sun C W, Wang L X, et al. Evaluation of field heat tolerance at seedling stage of Sorbus clone from different producing areas[J]. Journal of Beijing Forestry University, 2020, 42(4): 21−31. doi: 10.12171/j.1000-1522.20190266
[24] 苗胜越. 不同种源花楸越夏能力比较研究[D]. 保定: 河北农业大学, 2019. Miao S Y. Comparative study on the ability of Sorbus with different provenances[D]. Baoding: Hebei Agricultural University, 2019.
[25] 闫铭. 花楸树种子育苗关键技术研究[D]. 太谷: 山西农业大学, 2019. Yan M. Research on the key technology of sorbus tree seedling cultivation[D]. Taigu: Shanxi Agricultural University, 2019.
[26] 李建军. 几种花楸属植物繁殖技术研究进展[J]. 北方果树, 2017(1): 1−3. Li J J. Research progress on reproduction technology of several Sorbus plants[J]. Northern Fruit Tree, 2017(1): 1−3.
[27] 王雅婧. 山西省野生花楸的园林应用价值评价与繁殖方法研究[D]. 太谷: 山西农业大学, 2015. Wang Y J. Research on the garden application value evaluation and propagation methods of wild Sorbus in Shanxi Province[D]. Taigu: Shanxi Agricultural University, 2015.
[28] 肖乾坤, 张川红, 郑勇奇. 影响花楸树嫩枝扦插成活率关键因素分析[J]. 河北农业大学学报, 2010, 33(3): 67−71. doi: 10.3969/j.issn.1000-1573.2010.03.015 Xiao Q K, Zhang C H, Zheng Y Q. Analysis of key factors affecting the survival rate of tenderwood cuttings of Sorbus pohuashanensis[J]. Journal of Hebei Agricultural University, 2010, 33(3): 67−71. doi: 10.3969/j.issn.1000-1573.2010.03.015
[29] 赵洋, 陈云, 张晓晨, 等. 遮阴对少叶花楸幼苗光合和叶绿素荧光参数的影响[J]. 东北林业大学学报, 2019, 47(1): 30−34. doi: 10.3969/j.issn.1000-5382.2019.01.006 Zhao Y, Chen Y, Zhang X C, et al. Effects of shading on the photosynthesis and chlorophyll fluorescence parameters of Sorbus pohuashanensis seedlings[J]. Journal of Northeast Forestry University, 2019, 47(1): 30−34. doi: 10.3969/j.issn.1000-5382.2019.01.006
[30] 彭松, 郑勇奇, 马淼, 等. 高温胁迫下花楸树幼苗的生理响应[J]. 林业科学研究, 2011, 24(5): 602−608. Peng S, Zheng Y Q, Ma M, et al. Physiological response of Sorbus seedlings under high temperature stress[J]. Forestry Science Research, 2011, 24(5): 602−608.
[31] Zhang Z, Pei X, Zhang R, et al. Molecular characterization and expression analysis of small heat shock protein 17.3 gene from Sorbus pohuashanensis (Hance) Hedl. in response to abiotic stress[J]. Molecular Biology Reports, 2020, 47(12): 1−11.
[32] 张泽, 裴鑫, 鲁仪增, 等. 花楸树小热激蛋白23.8基因(SpHSP23.8)克隆与表达分析[J]. 植物资源与环境学报, 2020, 29(5): 9−20. doi: 10.3969/j.issn.1674-7895.2020.05.02 Zhang Z, Pei X, Lu Y Z, et al. Cloning and expression analysis of Sorbus pohuashanensis small heat shock protein 23.8 gene (SpHSP23.8)[J]. Journal of Plant Resources and Environment, 2020, 29(5): 9−20. doi: 10.3969/j.issn.1674-7895.2020.05.02
[33] 刘聪聪, 张泽, 关雪莲, 等. 花楸树热激蛋白70基因的克隆及表达分析[J]. 分子植物育种, 2019, 17(19): 6276−6286. Liu C C, Zhang Z, Guan X L, et al. Cloning and expression analysis of Sorbus pohuashanensis heat shock protein 70 gene[J]. Molecular Plant Breeding, 2019, 17(19): 6276−6286.
[34] Pei X, Zhang Y, Zhu L Y, et al. Physiological and transcriptomic analyses characterized high temperature stress response mechanisms in Sorbus pohuashanensis[J]. Scientific Reports, 2021, 11: 10117. doi: 10.1038/s41598-021-89418-7
[35] 吴丹丹. SlNAC8提高转基因拟南芥抗逆性及机制分析[D]. 大连: 辽宁师范大学, 2018. Wu D D. SlNAC8 improves stress resistance of transgenic Arabidopsis and its mechanism analysis[D]. Dalian: Liaoning Normal University, 2018.
[36] Sarkar N K, Kundnani P, Grover A. Functional analysis of Hsp70 superfamily proteins of rice (Oryza sativa)[J]. Cell Stress & Chaperones, 2013, 18(4): 427.
[37] Ingolia T D, Craig E A. Drosophila gene related to the major heat shock-induced gene is transcribed at normal temperatures and not induced by heat shock[J]. Proceedings of the National Academy of Science, 1982, 79(2): 525−529. doi: 10.1073/pnas.79.2.525
[38] 屠小菊. 拟南芥DBB1α基因热响应的分子机理及原核表达[D]. 长沙: 湖南大学, 2010. Tu X J. Molecular mechanism and prokaryotic expression of Arabidopsis DBB1α gene thermal response[D]. Changsha: Hunan University, 2010.
[39] 徐红云, 张明意. GRAS转录因子AtSCL4负调控拟南芥应答渗透胁迫[J]. 生物技术通报, 2022, 38(6): 129−135. Xu H Y, Zhang M Y. AtSCL4, an Arabidopsis thaliana GRAS transcription factor, negatively modulates plants in response to osmotic stress[J]. Biotechnology Bulletin, 2022, 38(6): 129−135.
[40] Liu H C, Liu H T, Charng Y Y. The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis[J]. Plant, Cell & Environment, 2011, 34(5): 738−751.
[41] Ikeda M, Mitsuda N, Ohme-Takagi M. Arabidopsis HsfB1 and HsfB2b act as repressors of the expression of heat-inducible Hsfs but positively regulate the acquired thermotolerance[J]. Plant Physiology, 2011, 157(3): 1243−1254. doi: 10.1104/pp.111.179036
[42] Tiwari L D, Khungar L, Grover A. AtHsc70-1 negatively regulates the basal heat tolerance in Arabidopsis thaliana through affecting the activity of HsfAs and Hsp101[J]. The Plant Journal, 2020, 103(6): 2069−2083. doi: 10.1111/tpj.14883
[43] 姚紫薇, 孙婧靓, 刘建祥, 等. 拟南芥热激转录因子HSFB2b负调控植物热形态建成[J/OL]. 浙江大学学报(农业与生命科学版), 2022. [2022−07−13]. http://kns.cnki.net/kcms/detail/33.1247.S.20220410.1723.002.Html. Yao Z W, Sun J L, Liu J X, et al. Heat shock transcription factor HSFB2b of Arabidopsis thaliana negatively regulates plant thermomorphogenesis. [J/OL]. Journal of Zhejiang University (Agriculture and Life Sciences ), 2022 [2022−07−13]. http://kns.cnki.net/kcms/detail/33.1247.S.20220410.1723.002.Html.
-
期刊类型引用(2)
1. 朱莉,王猛,孟兆新,李博,乔际冰. 基于强化学习的木工送料平台误差控制研究. 林产工业. 2023(11): 38-45 .  百度学术
百度学术
2. 孟兆新,郭骐瑞,邢鑫,殷鑫,宋绪秋. 基于数字孪生的并联式曲线送料平台误差分析. 林业机械与木工设备. 2022(02): 28-33 .  百度学术
百度学术
其他类型引用(1)



 下载:
下载: