Preparation of activated carbon from bamboo shoot shell by pyrolysis self-activation method and its arsenic adsorption performance
-
摘要:目的
利用竹材加工剩余物竹箨探索一种高效除砷活性炭材料。
方法以竹箨为原材料,以微波加热为热源,利用高温热解自活化技术在不同的活化温度和时间下制备竹箨活性炭,通过表征竹箨活性炭的微观形貌、比表面积、孔隙结构、石墨化程度、表面元素和官能团,揭示活化时间和温度等对其微观结构的影响,探讨竹箨活性炭的砷吸附性能,比较不同制备方法下活性炭的比表面积和砷吸附容量的差异。
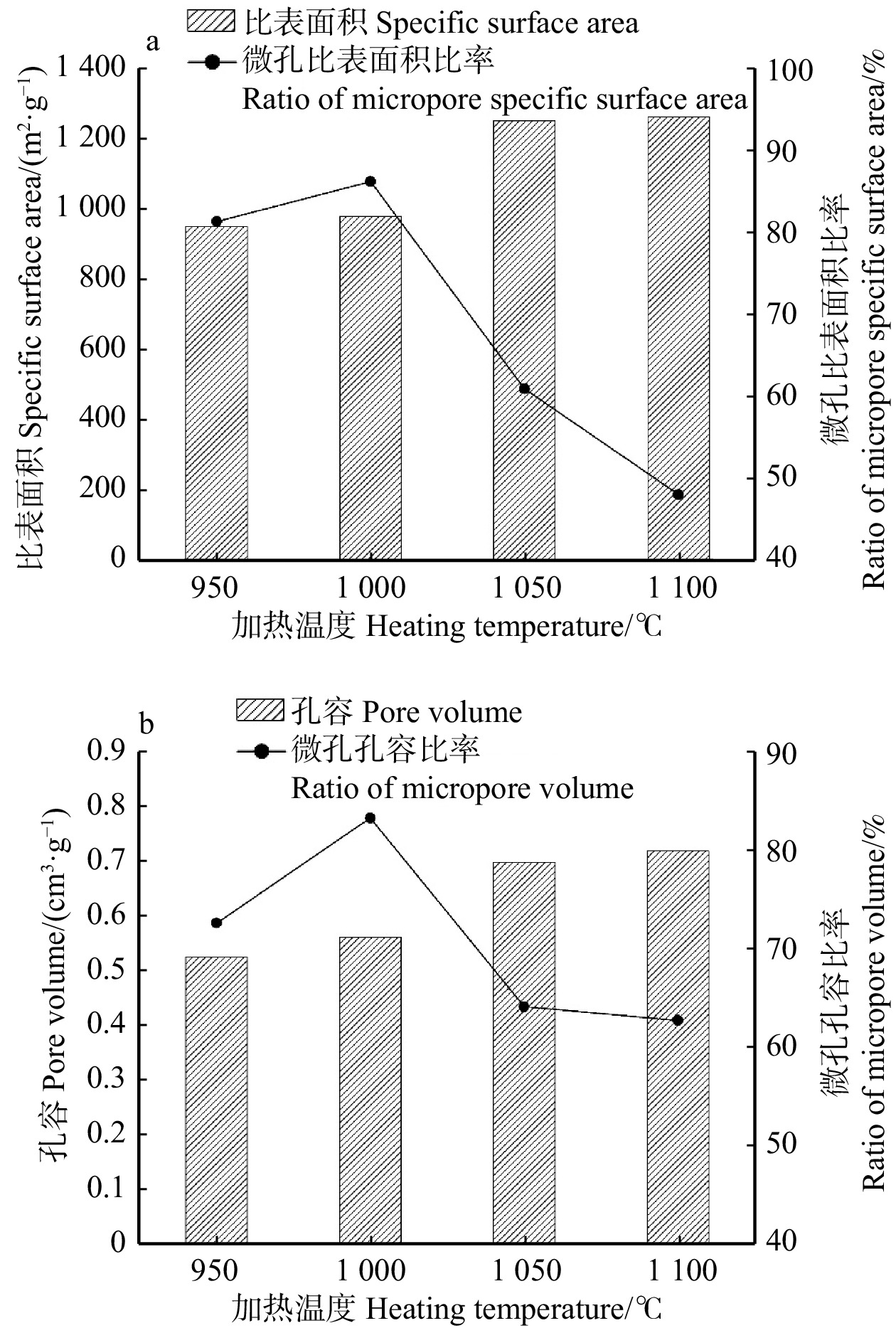
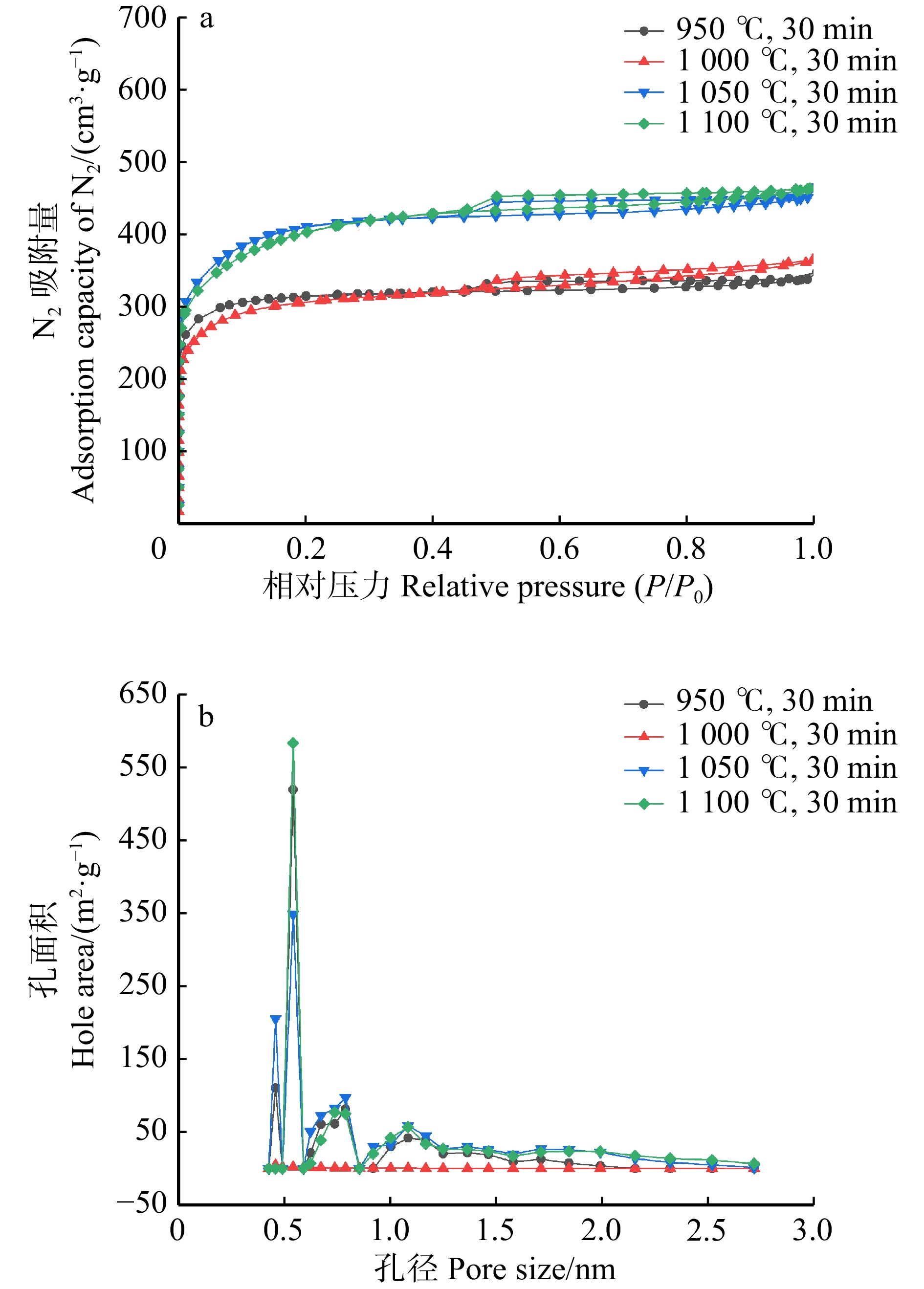
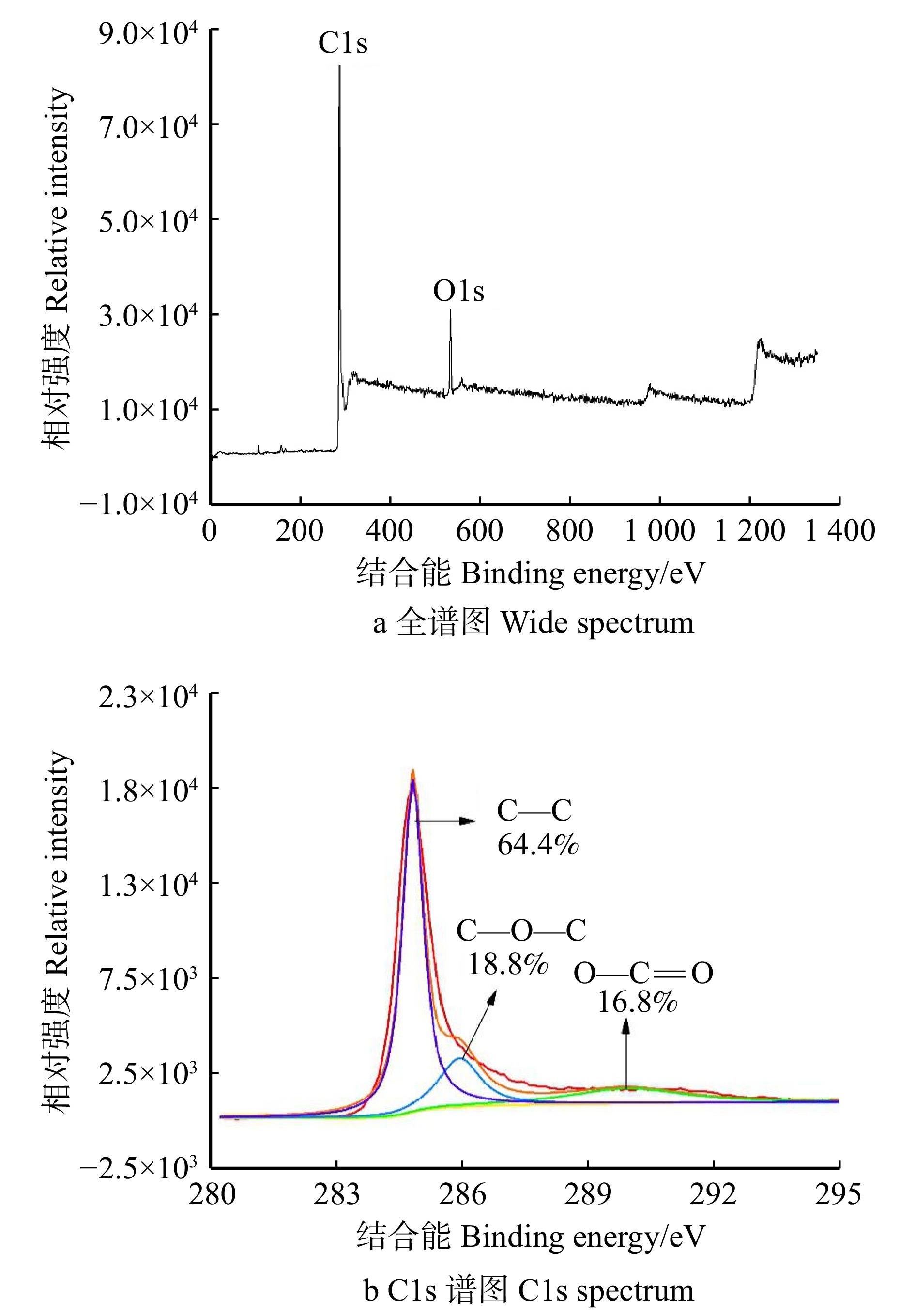
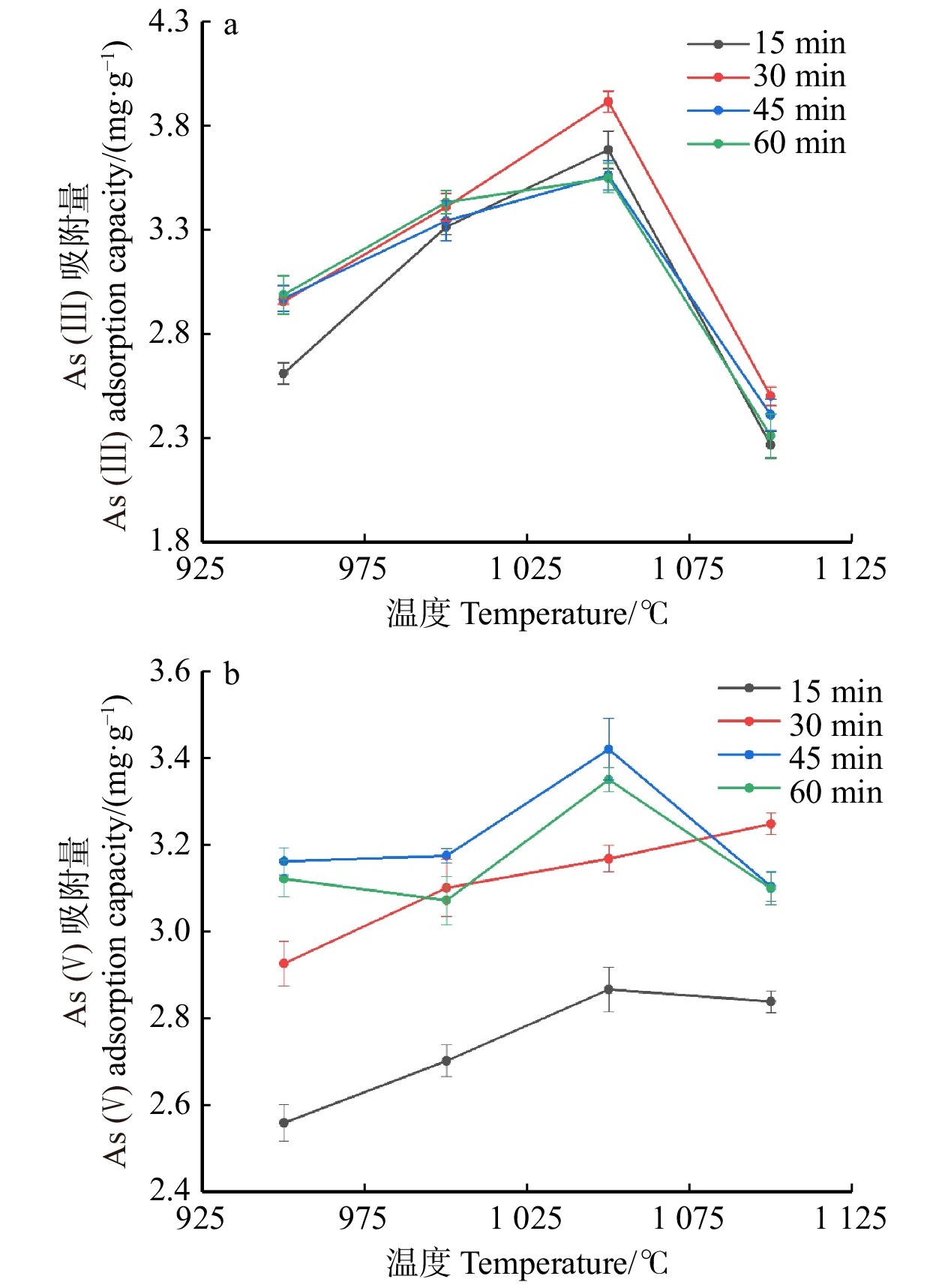
结果活化温度1 050 ℃、活化时间30 min时,竹箨活性炭孔隙结构排列整齐致密,比表面积达到1 251.7 m2/g,孔容为0.697 cm3/g,微孔比表面积比率和微孔孔容比率分别为60.9%和64.0%,平均孔径为0.448 nm,主要由微孔和少量介孔组成,孔径远大于砷酸根离子(AsO4 3−)和亚砷酸分子(H3AsO3)的空间构型尺寸,有利于对As(Ⅲ)和As(Ⅴ)的吸附。反映石墨化程度的R 值为1.340,表面具有丰富的含氧官能团,对As(Ⅲ)的最大吸附量为3.87 mg/g,对As(Ⅴ)的最大吸附量为3.17 mg/g。对比文献中不同活性炭的比表面积和砷吸附容量,竹箨活性炭表现出一定优势。
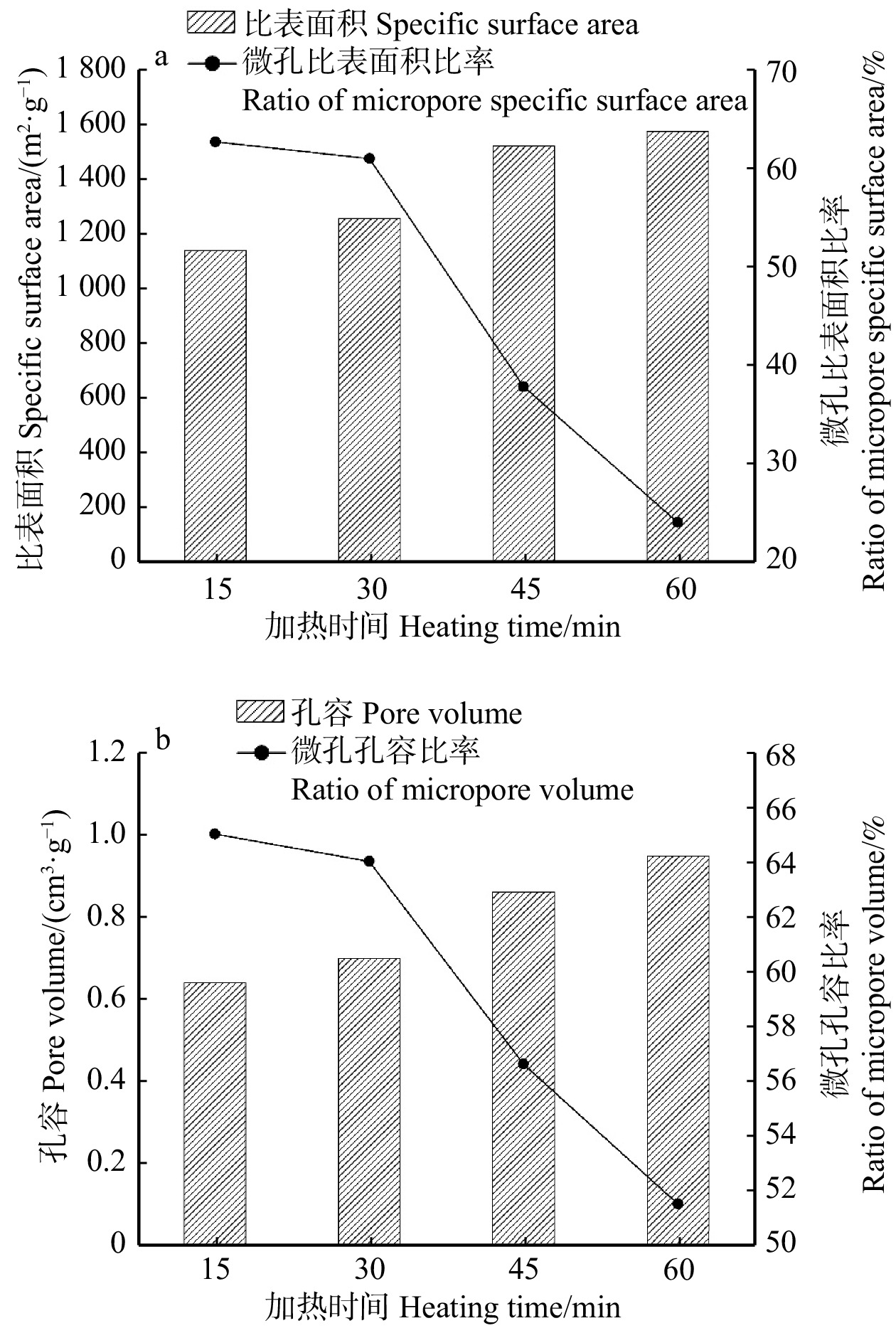
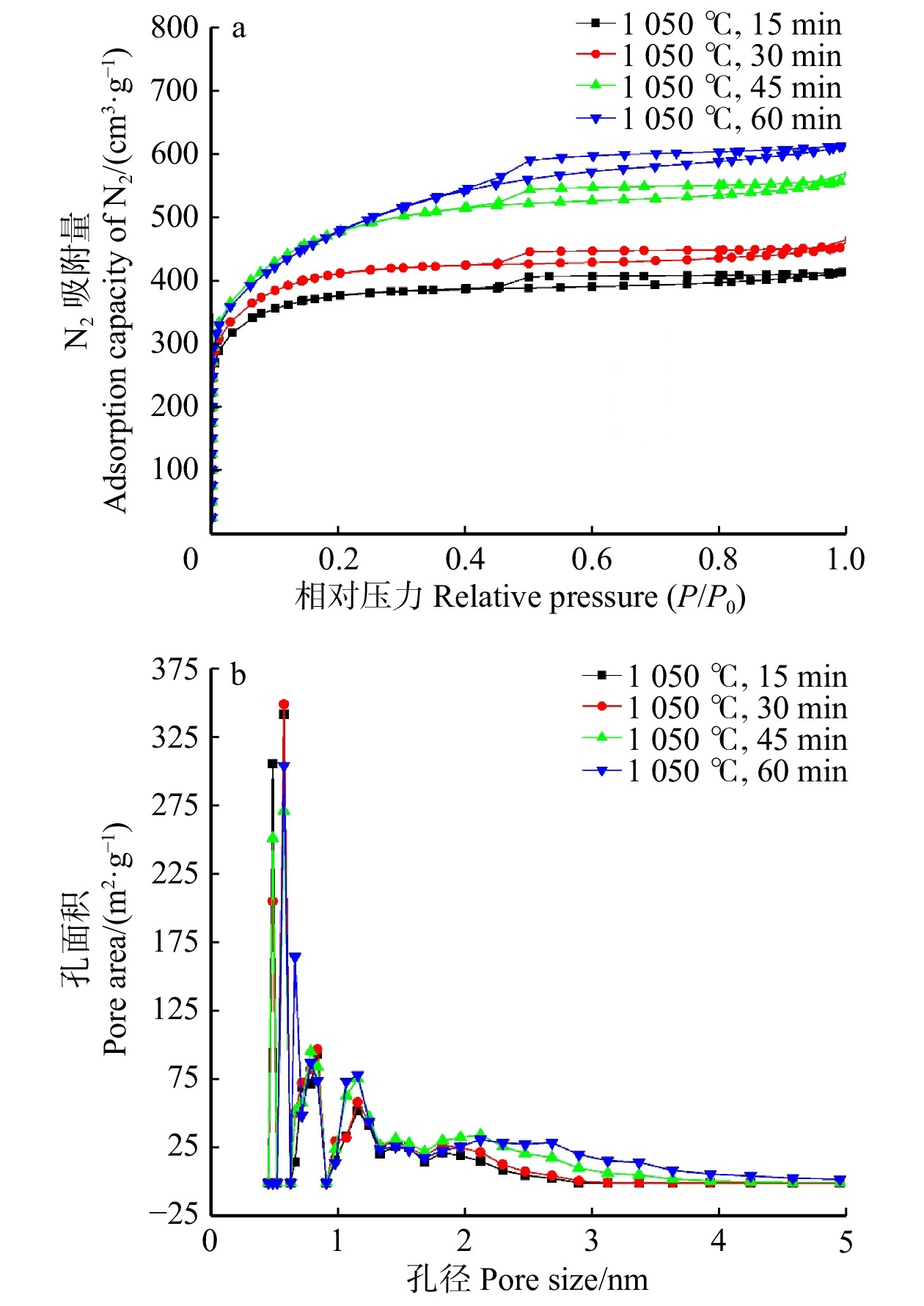
结论适当提高活化温度、延长活化时间有利于表面微孔的形成,从而提高砷吸附容量;但过高的活化温度和过长的活化时间会导致孔隙结构坍塌,减小比表面积和微孔比率,降低砷吸附容量。本研究为高效治理水体砷污染活性炭材料的制备提供了一种简单环保的方法,具有良好的除砷性能。
Abstract:ObjectiveThis paper aims to explore an efficient arsenic removal activated carbon material using bamboo shoot shell residue from bamboo processing.
MethodBamboo shoot shell was used as the raw material, while microwave heating was employed as the heat source. Activated carbon from bamboo shoot shell was prepared through catalytic pyrolysis self-activation technique at different activation temperatures and times. The microstructure, specific surface area, pore structure, graphitization degree, surface elements, and functional groups of the activated carbon were characterized to reveal the influence of activation time and temperature on its microstructure. The arsenic adsorption performance of the activated carbon was investigated, and the differences in specific surface area and arsenic adsorption capacity were compared among different preparation methods.
ResultAt an activation temperature of 1 050 ℃ and activation time of 30 min, the activated carbon exhibited an orderly and dense pore structure. The specific surface area reached 1 251.7 m2/g, with a pore volume of 0.697 cm3/g. The ratio of micropore specific surface area and the ratio of pore volume were 60.9% and 64.0%, respectively. The average pore diameter was 0.448 nm, primarily composed of micropores and a small amount of mesopores. The pore size was much larger than the spatial configuration dimensions of arsenate ions (AsO4 3−) and arsenous acid molecules (H3AsO3), which facilitated the adsorption of As(Ⅲ) and As(V). The graphitization degree (R) was 1.340, and the surface contained abundant oxygen-containing functional groups. The maximum adsorption capacity for As(Ⅲ) was 3.87 mg/g, while for As(V), it was 3.17 mg/g. Compared with the specific surface area and arsenic adsorption capacity of activated carbon in previous literature, the bamboo strip activated carbon demonstrated certain advantages.
ConclusionProperly increasing the activation temperature and extending the activation time are beneficial for the formation of surface micropores, thereby enhancing the arsenic adsorption capacity. However, excessively high activation temperature and prolonged activation time can cause the collapse of pore structure, resulting in reduced specific surface area, micropore ratio, and reduce arsenic adsorption capacity. This research provides a simple and environmentally friendly method for the preparation of efficient arsenic removal activated carbon materials, offering promising arsenic treatment performance in water bodies.
-
-
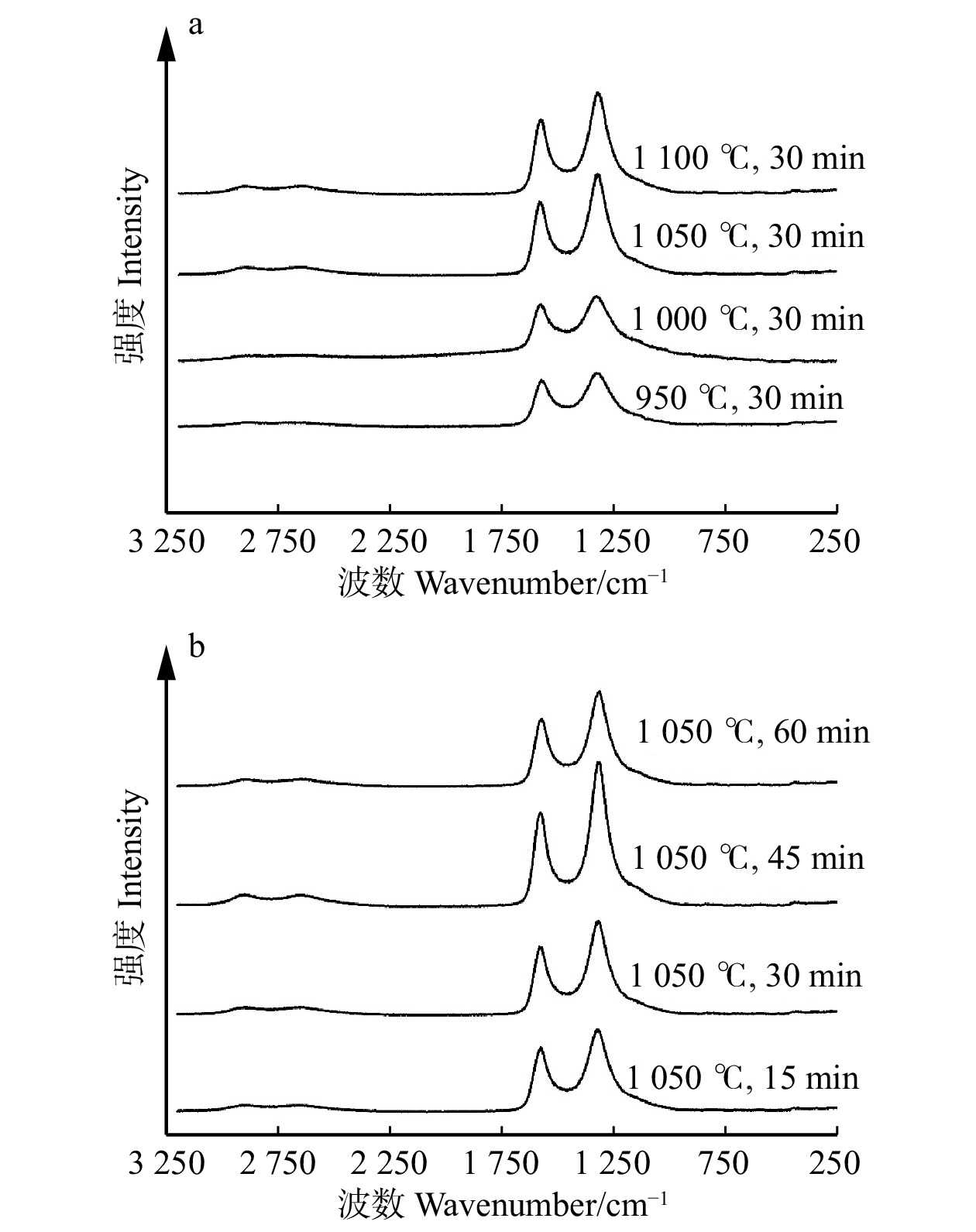
图 1 不同工艺条件下竹箨活性炭的SEM图
a. 950 ℃, 15 min; b. 950 ℃, 30 min; c. 950 ℃, 45 min; d. 950 ℃, 60 min; e. 1 000 ℃, 15 min; f. 1 000 ℃, 30 min; g. 1 000 ℃, 45 min; h. 1 000 ℃, 60 min; i. 1 050 ℃, 15 min; j. 1050 ℃, 30 min; k. 1 050 ℃, 45 min; l. 1 050 ℃, 60 min; m. 1 100 ℃, 15 min; n. 1 100 ℃, 30 min; o. 1 100 ℃, 45 min; p. 1 100 ℃, 60 min.
Figure 1. SEM images of activated carbon from bamboo shoot shell under different process conditions
表 1 不同加热温度下竹箨活性炭的比表面积和孔隙结构参数
Table 1 Specific surface area and pore structure of activated carbon from bamboo shoot shell at different heating temperatures
加热条件
Heating condition比表面积
Specific surface area/(m2·g−1)微孔比表面积比率
Ratio of micropore
specific surface area/%孔容
Pore volume/(cm3·g−1)微孔孔容比率
Ratio of micropore
volume/%平均孔径
Average pore
size/nm微孔
Micropore介孔
Mesoporous总和
Total微孔
Micropore介孔
Mesoporous总和
Total950 ℃,30 min 772.2 177.6 949.8 81.3 0.379 0.144 0.523 72.5 0.427 1 000 ℃,30 min 843.8 135.1 978.9 86.2 0.416 0.143 0.559 83.2 0.413 1 050 ℃,30 min 762.6 489.1 1 251.7 60.9 0.446 0.251 0.697 64.0 0.448 1 100 ℃,30 min 605.7 656.1 1 261.8 48.0 0.449 0.268 0.717 62.6 0.457 表 2 不同加热时间竹箨活性炭的比表面积和孔隙结构参数
Table 2 Specific surface area and pore structure of activated carbon from bamboo shoot shell at different heating times
样品
Sample比表面积
Specific surface area/(m2·g−1)微孔比表面积比率
Ratio of micropore
specific surface are/%孔容
Pore volume/(cm3·g−1)微孔孔容比率
Ratio of
micropore volume/%平均孔径
Average pore
size/nm合计
Total微孔
Micropore介孔
Mesoporous合计
Total微孔
Micropore介孔
Mesoporous1 050 ℃,15 min 1 136.3 711.3 245.0 62.6 0.638 0.415 0.223 65.0 0.438 1 050 ℃,30 min 1 251.7 762.6 489.1 60.9 0.697 0.446 0.251 64.0 0.448 1 050 ℃,45 min 1 517.8 572.9 944.9 37.7 0.859 0.486 0.373 56.6 0.463 1 050 ℃,60 min 1 571.4 375.5 1 195.9 23.9 0.947 0.488 0.461 51.5 0.471 表 3 活性炭砷吸附量对比研究
Table 3 Comparative study on arsenic adsorption capacity of activated carbon
样品
Sample比表面积
Specific surface
area/(m2·g−1)砷吸附量
Arsenic adsorption capacity/(mg·g−1)制备方法
Preparation method数据来源
Data sourceAs(Ⅲ) As(Ⅴ) 黄麻活性炭 Jute stick activated carbon 1 624 4.50 — H2O Asadullah等,2014[21]
Asadullah et al, 2014黄麻活性炭 Jute stick activated carbon 750 5.50 — H3PO4 载铁黄麻活性炭
Iron-loaded activated carbon1 266 9.70 — FeSO4 + H3PO4 颗粒活性炭
Granular activated carbon2.87 2.30 Fe + Mn Nikic等,2019[22]
Nikic et al, 2019竹箨活性炭
Activated carbon from bamboo shoot shell1 251.7 3.87 3.17 热解自活化
Pyrolysis self-activation本研究 Our research -
[1] Hong H J, Farooq W, Yang J S, et al. Preparation and evaluation of Fe-Al binary oxide for arsenic removal: comparative study with single metal oxides[J]. Separation Science and Technology, 2010, 45(12−13): 1975−1981. doi: 10.1080/01496395.2010.493790
[2] Marino T, Figoli A. Arsenic removal by liquid membranes[J]. Membranes (Basel), 2015, 5(2): 150−167. doi: 10.3390/membranes5020150
[3] Banerji T, Chaudhari S. A cost-effective technology for arsenic removal: case study of zerovalent iron-based IIT Bombay arsenic filter in West Bengal[M]//Nath K J, Sharma V P. Water and Sanitation in the New Millennium. New Delhi: Springer, 2017: 127−137.
[4] Hansen H K, Nuñez P, Raboy D, et al. Electrocoagulation in wastewater containing arsenic: comparing different process designs[J]. Electrochimica Acta, 2007, 52(10): 3464−3470. doi: 10.1016/j.electacta.2006.01.090
[5] Rathi B S, Kumar P S, Ponprasath R, et al. An effective separation of toxic arsenic from aquatic environment using electrochemical ion exchange process[J]. Journal of Hazardous Materials, 2021, 412: 125240. doi: 10.1016/j.jhazmat.2021.125240
[6] Liu C H, Chuang Y H, Chen T Y, et al. Mechanism of arsenic adsorption on magnetite nanoparticles from water: thermodynamic and spectroscopic studies[J]. Environmental Science & Technology, 2015, 49(13): 7726−7734.
[7] Li W G, Gong X J, Wang K, et al. Adsorption characteristics of arsenic from micro-polluted water by an innovative coal-based mesoporous activated carbon[J]. Bioresource Technology, 2014, 165: 166−173. doi: 10.1016/j.biortech.2014.02.069
[8] 陈延兴, 李建强, 严娜娜, 等. 竹笋壳纤维的提取与其基本结构特性[J]. 天津工业大学学报, 2009, 28(6): 32−34. doi: 10.3969/j.issn.1671-024X.2009.06.008 Chen Y X, Li J Q, Yan N N, et al. Extraction of bamboo shoot shell fiber and its basic structural characteristics[J]. Journal of Tianjin University of Technology, 2009, 28(6): 32−34. doi: 10.3969/j.issn.1671-024X.2009.06.008
[9] Hu H, Gao Y, Wang T, et al. Removal of hexavalent chromium, an analogue of pertechnetate, from aqueous solution using bamboo ( Acidosasa edulis) shoot shell[J]. Journal of Radioanalytical and Nuclear Chemistry, 2019, 321(2): 427−437. doi: 10.1007/s10967-019-06606-6
[10] Budinova T, Savova D, Tsyntsarski B, et al. Biomass waste-derived activated carbon for the removal of arsenic and manganese ions from aqueous solutions[J]. Applied Surface Science, 2009, 255(8): 4650−4657. doi: 10.1016/j.apsusc.2008.12.013
[11] Pallarés J, González-Cencerrado A, Arauzo I. Production and characterization of activated carbon from barley straw by physical activation with carbon dioxide and steam[J]. Biomass and Bioenergy, 2018, 115: 64−73. doi: 10.1016/j.biombioe.2018.04.015
[12] Mojitaba Z, Maryam O, Alireza M, et al. Competitive adsorption of arsenic and mercury on nano-magnetic activated carbons derived from hazelnut shell[J]. Korean Journal of Chemical Engineering, 2022, 39(2): 367−376. doi: 10.1007/s11814-021-0903-4
[13] Haghbin M R, Shahrak M N. Process conditions optimization for the fabrication of highly porous activated carbon from date palm bark wastes for removing pollutants from water[J]. Powder Technology, 2021, 377: 890−899. doi: 10.1016/j.powtec.2020.09.051
[14] Wu Y, Xia C, Cai L, et al. Controlling pore size of activated carbon through self-activation process for removing contaminants of different molecular sizes[J]. Journal of Colloid and Interface Science, 2018, 518: 41−47. doi: 10.1016/j.jcis.2018.02.017
[15] Xia C, Shi S Q. Self-Activatin process to fabricate activated carbon from kenaf[J]. Wood and Fiber Science, 2016, 48: 62−69.
[16] Sun K, Leng C Y, Jiang J C, et al. Microporous activated carbons from coconut shells produced by self-activation using the pyrolysis gases produced from them, that have an excellent electric double layer performance[J]. New Carbon Materials, 2017, 32(5): 451−459. doi: 10.1016/S1872-5805(17)60134-3
[17] Xia C, Shi S Q. Self-activation for activated carbon from biomass: Theory and parameters[J]. Green Chemistry, 2016, 18: 2063−2071. doi: 10.1039/C5GC02152A
[18] Rouquerol J, Avnir D, Fairbridge C W, et al. Recommendations for the characterization of porous solids[J]. Pure and Applied Chemistry, 1994, 66(8): 1739−1758. doi: 10.1351/pac199466081739
[19] Jayaramulu K, Dubal D P, Nagar B, et al. Ultrathin hierarchical porous carbon nanosheets for high-performance supercapacitors and redox electrolyte energy storage[J]. Advanced Materials, 2018, 30(15): 1705789. doi: 10.1002/adma.201705789
[20] Zou K, Deng Y, Chen J, et al. Hierarchically porous nitrogen-doped carbon derived from the activation of agriculture waste by potassium hydroxide and urea for high-performance supercapacitors[J]. Journal of Power Sources, 2018, 378: 579−588. doi: 10.1016/j.jpowsour.2017.12.081
[21] Asadullah M, Jahan I, Ahmed M B, et al. Preparation of microporous activated carbon and its modification for arsenic removal from water[J]. Journal of Industrial & Engineering Chemistry, 2014, 20(3): 887−896.
[22] Nikic J, Agbaba J, Watson M A, et al. Arsenic adsorption on Fe-Mn modified granular activated carbon (GAC-FeMn): batch and fixed-bed column studies[J]. Journal of Environmental Science and Health, Part A, 2019, 54(3): 168−178. doi: 10.1080/10934529.2018.1541375
-
期刊类型引用(1)
1. 徐媛,陈锦玲,陈玉梅,李璐璐,李惠敏,秦新民. 干旱胁迫下花生转录组与miRNA测序及相关基因的表达. 贵州农业科学. 2021(01): 1-9 .  百度学术
百度学术
其他类型引用(3)




 下载:
下载: