Effects of warming on soil microbial diversity and functional potential in alpine meadows
-
摘要:目的
本文旨在探究青藏高原高寒草甸生态系统中不同土壤微生物类群的多样性及其功能潜力对气候变暖的响应特征及机理。
方法基于为期6年的野外增温控制试验,利用不同功率红外加热设置对照、增低温(+1.5 ℃)和增高温(+2.5 ℃) 3个温度处理。增温6年后于2020年8月采集土壤表层样品,利用微生物高通量测序,分析土壤细菌和真菌群落对增温的响应。
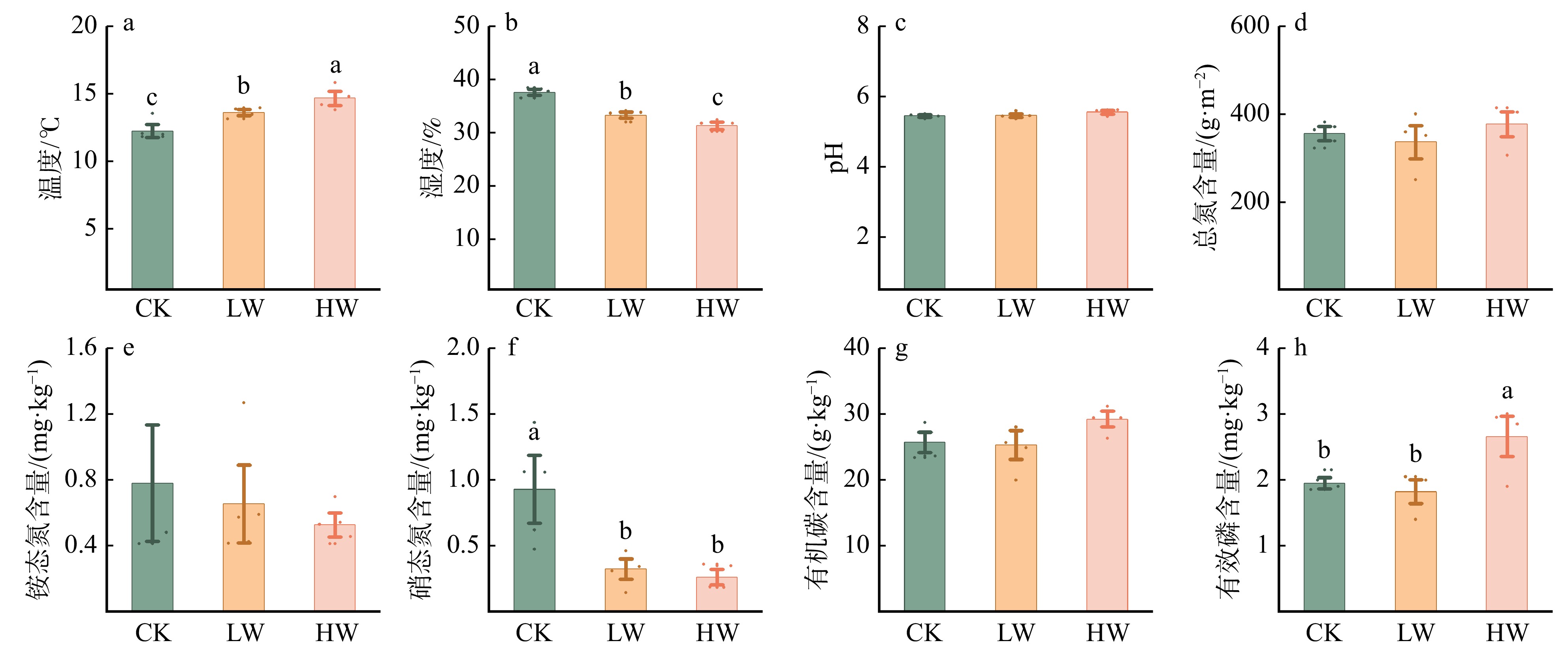
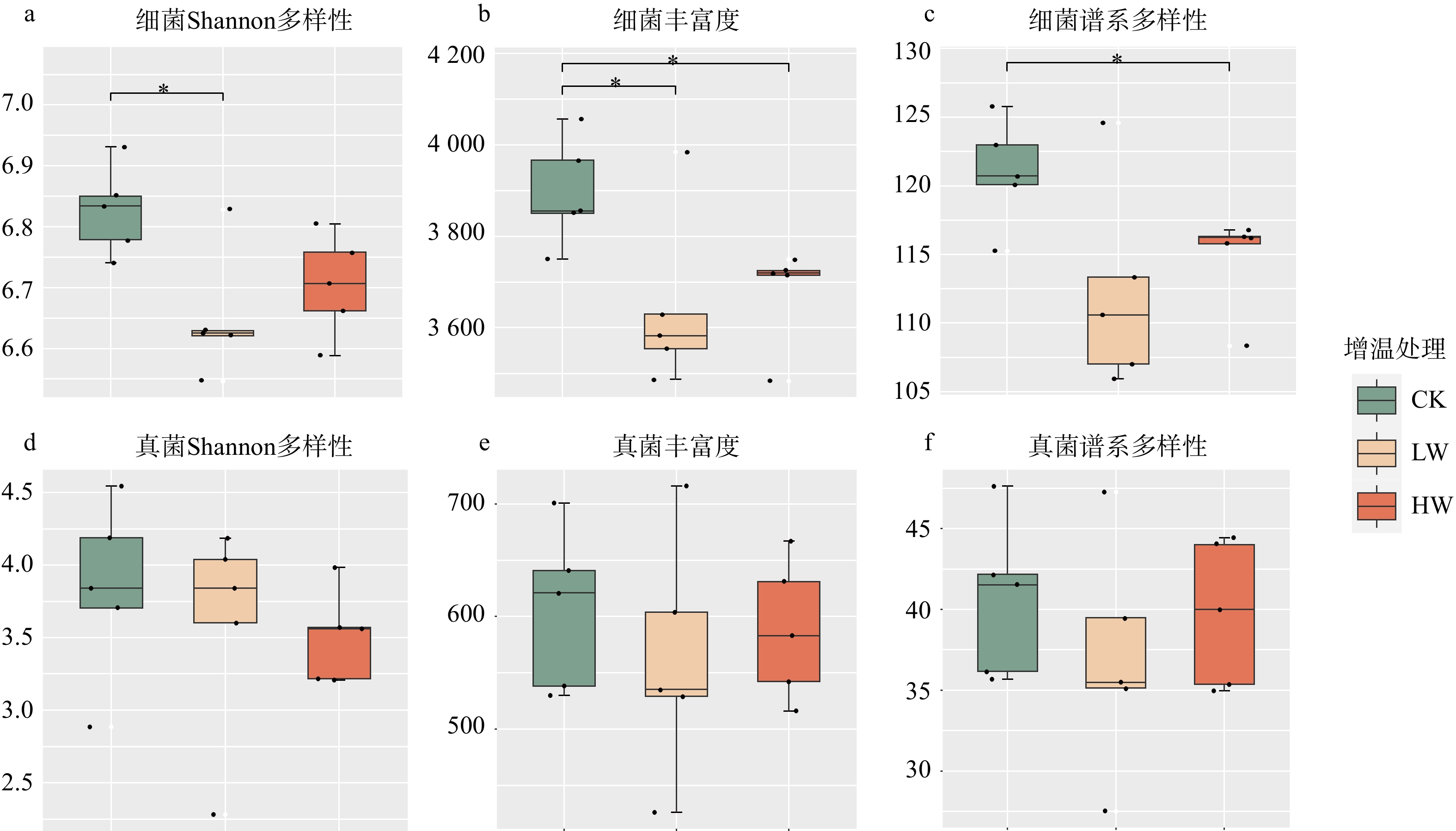
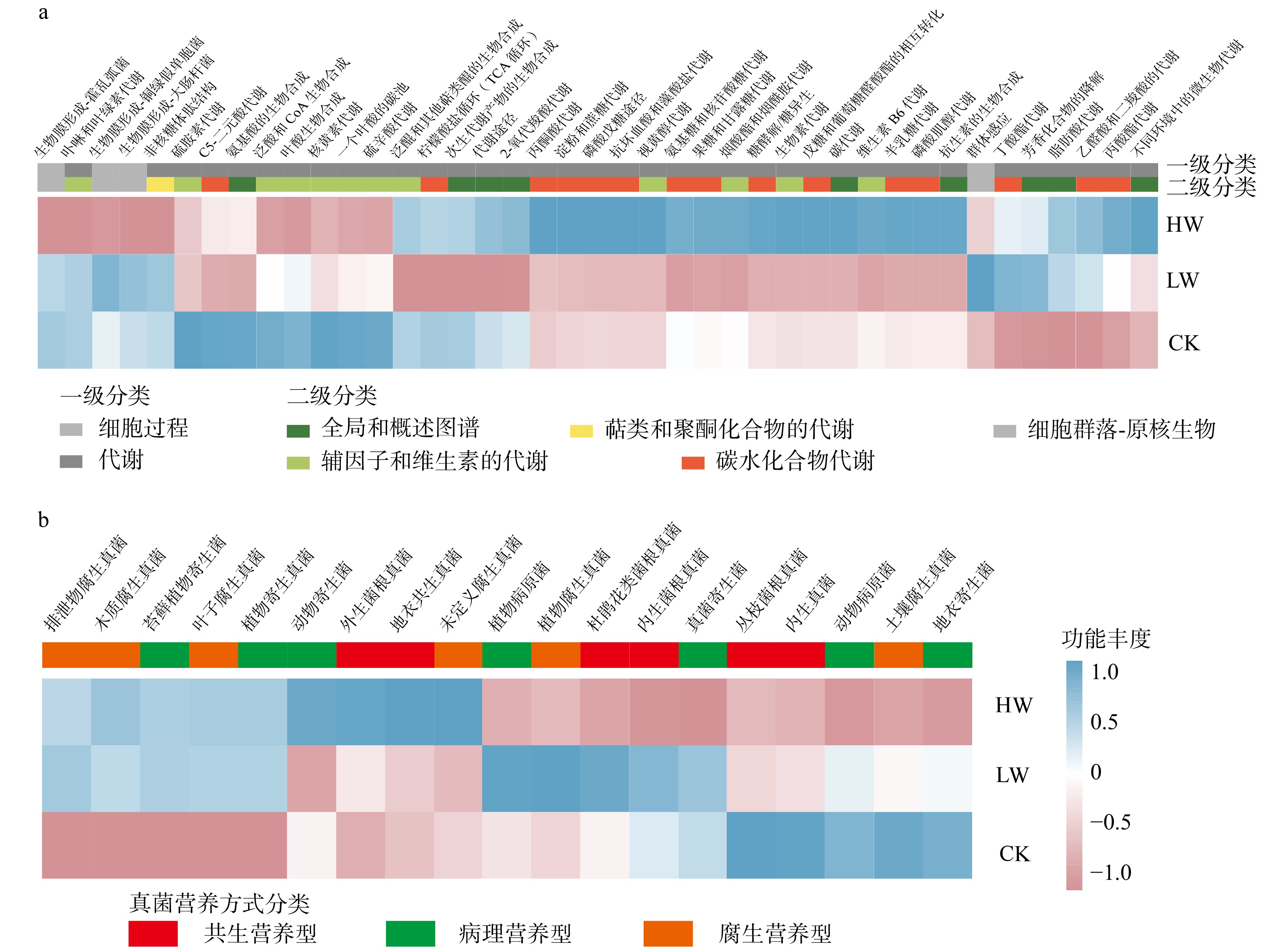
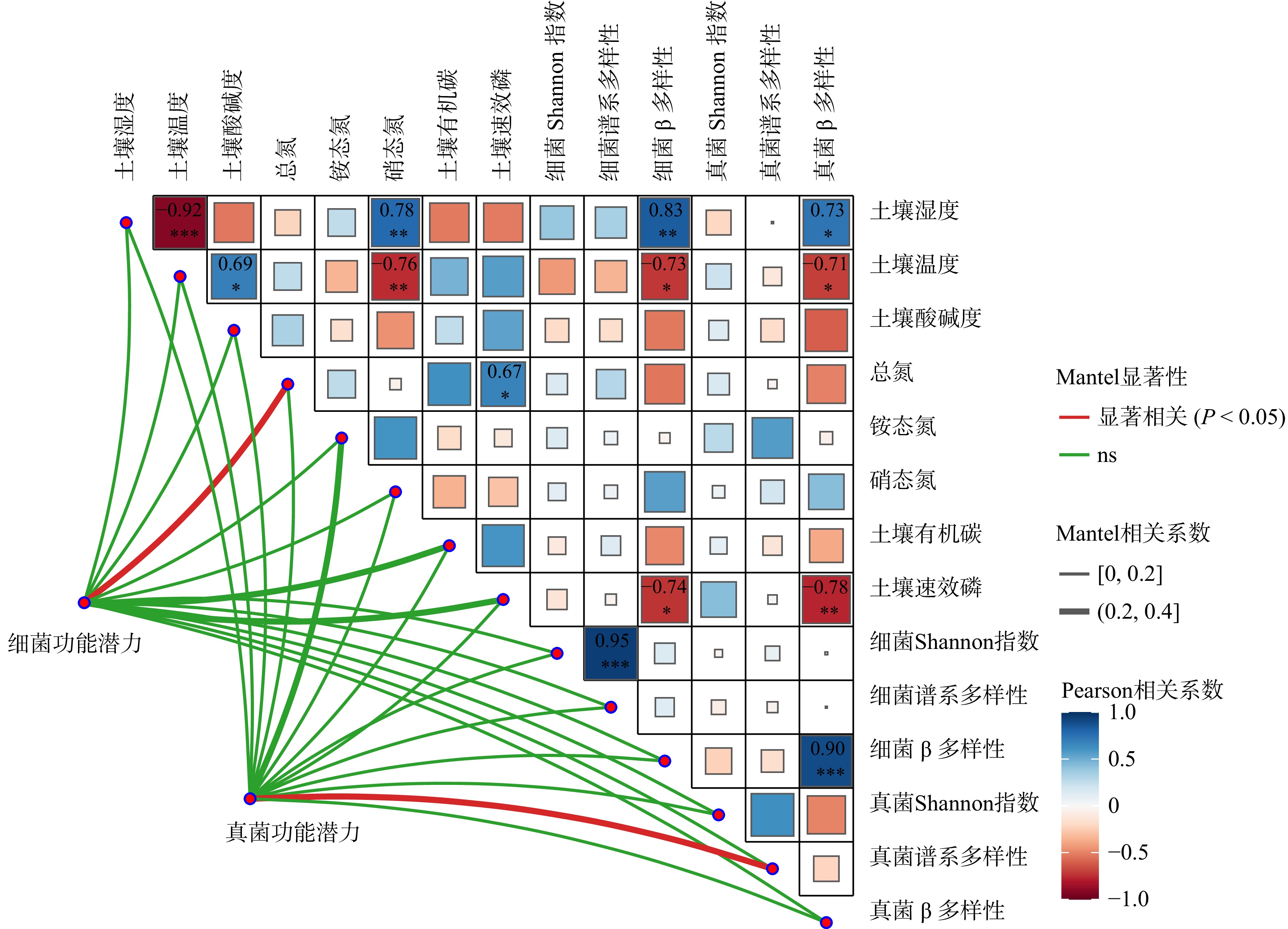
结果(1)增低温和增高温处理分别使土壤表层温度升高了1.4 ℃和2.4 ℃(P < 0.05),土壤湿度分别下降了11%和17%(P < 0.05)。相比对照,土壤硝态氮含量在增低温和增高温处理下分别降低了66%和72%(P < 0.05),而土壤有效磷含量仅在增高温处理下显著增加36%(P < 0.05)。(2)增温处理显著降低了土壤细菌α多样性及谱系多样性,并改变了细菌群落结构(P < 0.05);相比之下,土壤真菌以上指标均未表现出显著变化。(3)土壤细菌群落中,碳水化合物代谢及全局概览图等功能分类在增高温处理下明显增强,而土壤真菌共生营养型、病理营养型以及腐生营养型的功能丰度并未受显著影响。(4)土壤细菌功能潜力与土壤总氮含量和土壤有效磷含量呈显著正相关(P < 0.05),真菌功能潜力仅与谱系多样性呈显著正相关(P < 0.05),且与土壤理化性质无显著相关性。
结论增温显著降低了土壤细菌α多样性和谱系多样性,改变了细菌群落结构;增温通过调控土壤有效磷含量进而促进细菌群落在碳水化合物代谢等功能潜力的表达;相比之下,增温并未显著影响土壤真菌多样性、群落结构和功能潜力。研究结果表明土壤细菌群落与真菌群落对气候变暖响应的敏感性存在差异,可能对生态系统功能产生不同影响。
Abstract:ObjectiveThis study aimed to explore the response patterns of soil microbial diversity and their functional potentials to different warming levels in an alpine ecosystem on the Qinghai-Tibet Plateau of western China.
MethodWe conducted a field manipulation warming experiment using infrared heating methods to set three warming treatments, including control, low-level warming (+1.5 ℃) and high-level warming (+2.5 ℃). Through soil microbial high-throughput sequencing analysis, we investigated the general patterns and mechanisms underlying soil bacterial and fungi communities in response to field warming.
Result(1) Low-level warming and high-level warming significantly increased soil temperature in the topsoil by 1.4 and 2.4 ℃, and decreased soil moisture by 11% and 17%, respectively (P < 0.05). In contrast to the control, soil nitrate nitrogen content under low-level warming and high-level warming decreased by 66% and 72% (P < 0.05), respectively, while only high-level warming significantly stimulated soil available phosphorus content by 36% (P < 0.05). (2) Compared with control, warming significantly reduced soil bacterial α diversity and phylogenetic diversity, with notable differences in bacterial community structure (P < 0.05). But soil fungi community did not show any changes in these indicators. (3) In the soil bacterial community, functional categories such as carbohydrate metabolism and global overview map were significantly improved due to warming, while the abundance of three trophic types (i.e. symbiotroph, pathtroph and saprotroph) of soil fungi did not change under warming. (4) Soil bacterial functional potentials were mainly and positively correlated with soil total nitrogen and available phosphorus, while fungal functional potentials were positively associated with fungal Shannon diversity index.
ConclusionIn conclusion, warming significantly reduces soil bacterial α diversity and phylogenetic diversity, alters its β diversity. Warming promotes the expression of functional potentials such as carbohydrate metabolism in the bacterial community by regulating soil available phosphorous. In contrast, warming does not influence soil fungal diversity, community structure, and functional potentials. Our findings highlight the differential temperature sensitivities of soil bacterial and fungal communities, which may affect ecosystem functions differently.
-
Keywords:
- alpine meadow /
- bacteria /
- fungi /
- taxonomy diversity /
- phylogenetic diversity /
- function prediction
-
我国水果生产和消费能力均排名世界第一,但多数果园种植在丘陵山地,果树修整枝、疏花疏果、果实套袋和采收等作业主要依靠人工架梯来完成,劳动强度大、作业效率低、安全风险高[1-3],采摘器等辅助人工摘果装备同样存在作业效率低的问题[4-5]。丘陵山地地形地貌变化大,普通车辆很难进入,为了克服地形的影响,农业车辆多采用调平的方式来应对[6-9]。因此,设计研发一款可调平的果园升降作业平台对于提高果园机械化水平和作业效率,降低工作风险具有重要意义。
国外果园机械研究起步较早,北美自20世纪50年代开始把高空作业平台应用到果园中,发展至今已具有较高水平[10];意大利N.P.SEYMOUR公司生产的Windegger Picking Platform系列可全轮转向,工作台两级升降、角度可调,适用性较广[11];韩国SUNGBOO公司生产的SB系列采用纯电力能源,工作台可调角度10°,可满足缓丘陵作业需求[12-13]。
国内对于果园升降机械的研究起步较晚,主要因为果园种植模式以家庭分散种植为主,规模小,不利于果园机械的发展[14]。近些年,果园的主要种植模式为矮砧和密植,为果园机械化发展提供了前提条件。刘西宁等[15]研发了国内第一台果园升降平台,但不具备调平功能,不适合在丘陵山地作业。崔志超等[16]针对温室果蔬机械化低,传统燃油作业平台污染大等问题,设计制造了一款小型电动履带作业平台,采用电能作为动力,绿色环保,使用远程和在线的双操作系统。樊桂菊等[17-18]以拖拉机为载体加装工作台来实现调平,通过融合卡尔曼滤波的模糊PID控制系统实现自动调平,最大载荷150 kg,举升高度1.5 m,但是工作平台较小,在大规模果园作业时效率偏低。刘大为等[19-20]以南方丘陵山区柑橘园为对象研制了小型果园升降平台,通过“二次调平”方法,由液压缸伸缩进行调平,使用履带底盘,转向半径小、稳定性高、结构紧凑、操作简单,但调平方式为人工手动调平,调平精度低,而且作业效率不高。邱威等[21]根据中国南方果园多丘陵山区及果园作业的特点研制了一款折臂升降可调平平台,通过理论计算,仿真分析及样机试验分析验证了在丘陵山区的倾翻稳定性,并且利用正交试验分析了极限静态侧翻角的影响因子权重占比,在作业操作及相关机械的设计中有一定的借鉴意义。
国内公司现有果园产品多,但均不具备调平功能,无法适应丘陵山地果园作业,形成了“有机不好用”的局面,丘陵果园综合机械化水平仅为5.75%[2]。因此,针对丘陵山地矮砧密植苹果园地形与种植模式特点,研究升降平台调平机理,设计一款可调平的果园自走式升降作业平台,以期满足丘陵山地作业需求。
1. 整机结构与原理
1.1 设计要求
丘陵果园因地形特点,其种植模式主要有依山就势的缓坡模式和“坡改梯”的梯田模式两种。在这类果园中作业和转场对农业车辆的动力底盘要求很高,应具备良好的通过性和爬坡能力,本机选用越障能力强、转弯半径小的履带底盘。经查阅文献[22]和实地观测,苹果园一般株距1.0 ~ 1.5 m,行距3.5 ~ 4.0 m,树高不超过3.5 m。结合实际生产过程,可调平平台具体设计要求如表1所示。
表 1 果园作业平台设计参数Table 1. Design parameters of orchard operating platform参数 Parameter 数值 Value 整机尺寸 Overall size 1 900 mm × 1 400 mm × 2 000 mm 整机质量 Overall mass/kg 1 200 最大载质量 Maximum load/kg 300 升降高度 Lifting height/mm 0 ~ 1 150 转弯半径 Turning radius/m 3 行驶速度 Drving speed/(km·h−1) 0 ~ 5 1.2 整机结构
整机结构主要包括护栏、工作台、俯仰调平机构、机架、侧倾调平机构、履带底盘、操作台、剪叉机构和动力集成(图1)。动力集成安装在机架上,由电机、电池、液压管道和液压阀组成,为平台的升降、调平和行走等动作提供动力,并通过操作台控制平台升降和行走。工作台两侧可扩展,以增加工作平台面积,方便果农采摘套袋等作业。以下对平台的升降和调平机理做详细阐述。
1.3 工作台升降及俯仰调平原理
1.3.1 整体结构设计
因剪叉式升降机构具有较高的稳定性和承载能力[23-24],同时考虑到缓丘陵果园地形特点(坡度 ≤ 15°),以及矮砧密植型苹果树高一般不超过3.5 m,结合GB10000—1988《中国成年人人体尺寸标准》[25]可知平台升降高度达2 m即可满足果园作业要求,故选择单级剪叉升降机构,并在此基础上改进为折臂剪叉式俯仰调平机构(图2)。升降液压缸PQ伸长,工作台随之上升,反之工作台下降;俯仰液压缸MN伸长,俯仰臂O1D绕O1点旋转,工作台DE仰起一定角度θ(如图2中红色部分),反之工作台俯下一定角度−θ,达到俯仰调平目的。该调平方式没有额外增加过多机构,节省空间、简单可靠。
![]() 图 2 升降及俯仰调平机构原理O、O1分别为升降、俯仰调平机构坐标系原点;DE为工作台,PQ为升降液压缸,MN为俯仰液压缸;OD和EH为剪叉臂,其中OB、BD、EB和BH长度相等(mm);A、C两点为升降液压缸上下两端与剪叉臂的连接点,且AP与EH垂直,CQ与OB垂直;O和E分别为剪叉臂与机架、工作台的铰接点;D、H两点为铰接滑轮,滑轮与机架、工作台的滑轨相切,B点是两剪叉臂铰接点;h为工作台升起高度(mm);G为工作台和被载物体的总重(N);α为剪叉臂与水平面夹角(°);β为MO1与O1B的夹角(°);φ为俯仰臂O1D绕O1点转动的角度(°);θ为工作台与水平面夹角,即平台俯仰角(°);F1为升降液压缸推力(N);F2为俯仰液压缸推力(N)。O is the origin of lifting coordinate system, and O1 is the origin of pitch leveling mechanism coordinate system. DE is the platform, PQ is lifting hydraulic cylinder, MN is pitch hydraulic cylinder; OD and EH are scissor arms, and the lengths of OB, BD, EB and BH are equal (mm). A is the connection point between the upper end of lifting hydraulic cylinder and scissor arm, C is connection point between the lower end of lifting hydraulic cylinder and scissor arm, and AP is perpendicular to EH, and CQ is perpendicular to OB. O is hinge point between scissor arm and frame, E is the hinge point of scissor arm and platform; D and H are hinged pulleys, the pulley is tangent to slide rail of the rack and platform, B is hinge point of two scissor arms. h is lifting height of platform (mm). G is total mass of platform and the loaded object (N). α is the angle between scissor arm and horizontal plane (°). β is the angle between MO1 and O1B (°). φ is the angle that tilt arm O1D rotates around O1 point (°). θ is platform pitch angle between platform and horizontal plane (°). F1 is the thrust of lifting hydraulic cylinder (N). F2 is thrust of pitch hydraulic cylinder (N).Figure 2. Principles of lifting and pitch leveling mechanism
图 2 升降及俯仰调平机构原理O、O1分别为升降、俯仰调平机构坐标系原点;DE为工作台,PQ为升降液压缸,MN为俯仰液压缸;OD和EH为剪叉臂,其中OB、BD、EB和BH长度相等(mm);A、C两点为升降液压缸上下两端与剪叉臂的连接点,且AP与EH垂直,CQ与OB垂直;O和E分别为剪叉臂与机架、工作台的铰接点;D、H两点为铰接滑轮,滑轮与机架、工作台的滑轨相切,B点是两剪叉臂铰接点;h为工作台升起高度(mm);G为工作台和被载物体的总重(N);α为剪叉臂与水平面夹角(°);β为MO1与O1B的夹角(°);φ为俯仰臂O1D绕O1点转动的角度(°);θ为工作台与水平面夹角,即平台俯仰角(°);F1为升降液压缸推力(N);F2为俯仰液压缸推力(N)。O is the origin of lifting coordinate system, and O1 is the origin of pitch leveling mechanism coordinate system. DE is the platform, PQ is lifting hydraulic cylinder, MN is pitch hydraulic cylinder; OD and EH are scissor arms, and the lengths of OB, BD, EB and BH are equal (mm). A is the connection point between the upper end of lifting hydraulic cylinder and scissor arm, C is connection point between the lower end of lifting hydraulic cylinder and scissor arm, and AP is perpendicular to EH, and CQ is perpendicular to OB. O is hinge point between scissor arm and frame, E is the hinge point of scissor arm and platform; D and H are hinged pulleys, the pulley is tangent to slide rail of the rack and platform, B is hinge point of two scissor arms. h is lifting height of platform (mm). G is total mass of platform and the loaded object (N). α is the angle between scissor arm and horizontal plane (°). β is the angle between MO1 and O1B (°). φ is the angle that tilt arm O1D rotates around O1 point (°). θ is platform pitch angle between platform and horizontal plane (°). F1 is the thrust of lifting hydraulic cylinder (N). F2 is thrust of pitch hydraulic cylinder (N).Figure 2. Principles of lifting and pitch leveling mechanism1.3.2 升降和俯仰液压缸推力分析
根据图2建立液压缸推力、俯仰液压缸位移量和平台角度关系式,以确定优化液压缸所需推力和平台尺寸。由图2可知相关量的几何关系:
{xPQ=−lCQsinα+lBCcosα−lABcosα+lAPsinαyPQ=lCQcosα+lBCsinα+lABsinα+lAPcosαlPQ=√xPQ2+yPQ2h=lEHsinα (1) 式中:xPQ、yPQ分别为升降液压缸PQ在OX、OY轴上的投影长度(mm);lCQ、lBC、lAB和lAP分别为升降机构部分CQ、BC、AB和AP的长度(mm);lPQ为升降液压缸PQ的长度(mm);lEH为剪叉臂EH的长度(mm);α为剪叉臂与水平面夹角(°)。
根据虚位移原理[26]:
F1dlPQdα=Gdhdα (2) 得到:
F1=G2lEHcosα√asin2α+bsin2α+ccos2α(a−c)sin2α+2bcos2α (3) 式中:F1为升降液压缸推力(N);
a=(lAB+lBC)2+(lAP−lCQ)2 ,b=2(lABlCQ+lBClAP) ,c=(lBC−lAB)2+(lAP+lCQ)2 。由式(3)可知:F1不仅与lEH、lPQ、lBC、lAB和lAP有关,还与平台升降高度α有关,并且随着α的减小,液压缸所需推力随之增大。在工作台降到最低位置时,液压缸所需推力最大。
同理,在图2中以X1O1Y1为坐标系,根据虚位移原理可知:
F2=GlO1Dcos(α+φ)√lO1M2+lO1N2+2lO1MlO1Ncos(φ−β)2lO1MlO1Nsin(β−φ) (4) 式中:F2为俯仰液压缸推力(N);
lO1D 、lO1M 和lO1N 分别为俯仰调平机构部分O1D、O1M、O1N的长度(mm);β为MO1与O1B的夹角(°);φ 为俯仰臂O1D绕O1点转动的角度(°)。由式(4)可知:俯仰液压缸所需推力和平台俯仰角及平台升降高度有关,平台高度越低,俯仰角越大,所需推力越大,在平台降到最低且俯仰液压缸完全伸出时所需推力最大。考虑到工作台不能与底部动力集成干涉以及对升降高度的要求,对参数值选取合适的值。详细尺寸数据见表2。
表 2 升降及俯仰调平机构尺寸Table 2. Dimensions of lifting and pitch leveling structures参数 Parameter 数值 Value lAB/mm 220 lBC/mm 702 lAP/mm 70 lCQ/mm 97 lEH/mm 1 722 lO1N/mm 411 lO1M/mm 163 lO1D/mm 561 β/(°) 88 注:lAB、lBC、lAP和lCQ分别为升降机构AB、BC、AP和CQ的长度;lEH为剪叉臂EH的长度;lO1N、lO1M和lO1D分别为俯仰调平机构部分O1N、O1M和O1D的长度;β为MO1与O1B的夹角。Notes:lAB, lBC, lAP and lCQ are length of lifting mechanism AB, BC, AP and CQ, respectively. lEH is the length of scissor arm EH. lO1N, lO1M and lO1D are length of pitch leveling mechanism O1N, O1M and O1D, respectively. β is angle between MO1 and O1B. 1.3.3 平台俯仰调平倾角
在XOY坐标中:
{xOD1=lOO1cosα+lO1D1cos(α+φ)yOD1=lOO1sinα+lO1D1sin(α+φ) (5) 式中:
xOD1 、yOD1 分别为剪叉臂OD1在OY、OX轴上的投影长度(mm),lOO1 和lO1D1 分别为剪叉臂OO1和O1D1的长度(mm)。因为
tanθ=yOD1−hxOD1 ,可以得到:θ=arctan(lOO1−lEH)sinα+lO1D1sin(α+φ)lOO1cosα+lO1D1cos(α+φ) (6) 其中:
φ=arccoslO1M2+lO1N2−(lMN−y)22lO1MlO1N (7) 式中:y为俯仰液压缸活塞杆位移量(mm),
lMN 为俯仰液压缸MN的长度(mm),θ为工作台与水平面夹角,即平台俯仰角(°)。由式(6)和式(7)可知折臂剪叉俯仰调平机构可调角度与升降液压缸、俯仰液压缸的位移量有关。当平台降到最低点位置时,俯仰液压缸不能缩短,只能伸长。即平台只能进行仰调平,不能进行俯调平,否则工作台会与机架干涉。随着工作台的升起,工作台既能仰也能俯,可调角度均为10°。但工作台升到最高点时仰调平角度降至6.5°。
1.4 侧倾调平原理
1.4.1 侧倾调平机构设计
在履带底盘和机架之间设计了侧倾调平机构,起到连接和调平作用,机构简图如图3。液压缸I1J1和IJ做相反运动,机架O3J1J绕O3点旋转,工作台侧倾一定角度θ1,达到侧倾调平目的。
![]() 图 3 侧倾调平机构原理I1J1、IJ为侧倾液压缸;α1为侧倾液压缸I1J1与水平面的夹角(°);β1为侧倾液压缸IJ与水平面的夹角(°);θ1为平台与水平面的夹角,即平台侧倾角(°);O2为原点,I、I1、O3为底盘上的固定点;O3J1J为机架;O3R1与侧倾液压缸I1J1垂直,O3R与侧倾液压缸IJ垂直;e为机架上方总重G1在X2轴平行方向上与O2的距离,(mm);F3、F4为侧倾液压缸的推力(N)。I1J1 and IJ are the roll hydraulic cylinders, α1 is angle between roll hydraulic cylinder I1J1 and the horizontal plane (°). β1 is angle between roll hydraulic cylinder IJ and horizontal plane (°). θ1 is the angle between platform and horizontal plane, i.e. the platform roll angle (°). O2 is the origin, I,I1 and O3 are the fixed points on chassis, O3J1J is frame. O3R1 is perpendicular to the roll cylinder I1J1, O3R is perpendicular to roll cylinder IJ. e is the distance between total mass G1 and O2 in the direction parallel to X2 axis. F3 and F4 are thrust of roll hydraulic cylinder (N).Figure 3. Principle of roll leveling mechanism
图 3 侧倾调平机构原理I1J1、IJ为侧倾液压缸;α1为侧倾液压缸I1J1与水平面的夹角(°);β1为侧倾液压缸IJ与水平面的夹角(°);θ1为平台与水平面的夹角,即平台侧倾角(°);O2为原点,I、I1、O3为底盘上的固定点;O3J1J为机架;O3R1与侧倾液压缸I1J1垂直,O3R与侧倾液压缸IJ垂直;e为机架上方总重G1在X2轴平行方向上与O2的距离,(mm);F3、F4为侧倾液压缸的推力(N)。I1J1 and IJ are the roll hydraulic cylinders, α1 is angle between roll hydraulic cylinder I1J1 and the horizontal plane (°). β1 is angle between roll hydraulic cylinder IJ and horizontal plane (°). θ1 is the angle between platform and horizontal plane, i.e. the platform roll angle (°). O2 is the origin, I,I1 and O3 are the fixed points on chassis, O3J1J is frame. O3R1 is perpendicular to the roll cylinder I1J1, O3R is perpendicular to roll cylinder IJ. e is the distance between total mass G1 and O2 in the direction parallel to X2 axis. F3 and F4 are thrust of roll hydraulic cylinder (N).Figure 3. Principle of roll leveling mechanism1.4.2 侧倾液压缸推力分析
由图3可知:
{∠IO3R=β1−∠IO3O2lO3R=lIO3cos∠IO3R∠I1O3R1=α1−∠I1O3O2lO3R1=lI1O3cos∠I1O3R1F3lO3R+F4lO3R1=G1e (8) 式中:
lO3R 、lIO3 、lO3R1 和lI1O3 分别为侧倾调平机构部分O3R、IO3、O3R1和I1O3的长度(mm);F3、F4为侧倾液压缸的推力(N);∠IO3R为IO3与O3R的夹角(°);∠IO3O2为IO3与O3O2的夹角(°);∠I1O3R1为I1O3与O3R1的夹角(°);∠I1O3O2为I1O3与O3O2的夹角(°);G1为机架上方总重(N);e为G1在X2轴平行方向上与O2的距离(mm)。根据选用的启创液压科技公司HSG-40系列工程油缸得1.45F3 = F4,则:
F4=G10.6elIO3cos(β1−∠IO3O2)+lI1O3cos(α1−∠I1O3O2) (9) 从式(9)中可以看出G1的增加及因G1升高引起的e增加会导致液压缸推力的增加,在设计中对工作台零部件轻量化,降低重心,以减小液压缸所需推力,同时也能增加平台的侧倾稳定性。
1.4.3 平台侧倾调平倾角
由图3中关系可知:
θ1=arctanlIJsinβ1−lI1J1sinα1lI1J1cosα1+lI1I+lIJcosβ1 (10) 式中:
lIJ 、lI1J1 和lI1I 分别为IJ、I1J1和I1I的长度(mm);α1为侧倾液压缸I1J1与水平面的夹角(°);β1为侧倾液压缸IJ与水平面的夹角(°);θ1为平台与水平面的夹角,即平台侧倾角(°)从式(10)中可以看出侧倾液压缸的长度和安装位置是影响平台调平角度的主要因素。结合设计要求与结构特点选取合适的尺寸,侧倾角度达 ± 8°。具体尺寸数据见表3。
表 3 侧倾调平结构尺寸Table 3. Dimensions of roll leveling structures参数 Parameter 数值 Value α1/(°) 28 ~ 70.5 lIO3/mm 264 lIJ/mm 320 ~ 390 lI1I/mm 373 注:α1为侧倾液压缸I1J1与水平面的夹角;lIO3为侧倾调平机构部分IO3的长度;lIJ和lI1I分别为IJ和I1I的长度。Notes: α1 is the angle between roll hydraulic cylinder I1J1 and horizontal plane. lIO3 is length of tilting leveling mechanism IO3. lIJ and lI1I are the lengths of IJ and I1I, respectively. 2. 控制系统仿真
2.1 系统数学模型建立
升降、俯仰和侧倾3个控制回路组成整个系统,且具有相似的结构形式。因升降部分需手动控制,故只分析俯仰、侧倾控制系统。控制系统由控制器、放大器、电液比例阀、液压缸、倾角传感器等组成。
2.1.1 放大器传递函数
电路部分看作时间常数很小的放大器,可以作为比例环节[27],所以放大器传递函数为
G1(s)=I(s)Θ(s)=Ke1 (11) G2(s)=I(s)Θ(s)=Ke2 (12) 式中:I为电流(A),Θ为平台角度与目标角度差值(°),Ke1、Ke2分别为俯仰、侧倾部分放大器比例系数,G1(s)和G2(s)分别为俯仰、侧倾调平机构的放大器传递函数,s代表时间函数经拉普拉斯变换为复变函数(下同)。
2.1.2 电液比例阀数学模型
选用北京尚泰合力液压科技公司生产固有角频率69 rad/s的STPLS系列电液比例阀。建立电液比例阀的传递函数时,如果系统中存在固有角频率较大的动力元件选用二阶震荡环节[28-29]。所以电液比例阀的传递函数为:
G3(s)=Xv(s)I(s)=Kvs2ωv2+2ξvωvs+1 (13) 式中:ωv为比例阀固有角频率(rad/s),
ξv 为比例阀阻尼比,Xv为比例阀芯位移(m),Kv比例阀增益。2.1.3 液压缸模型建立
选用启创液压科技公司的HSG-40系列工程油缸,属于阀控非对称缸,其传递函数[30]为:
G4(s)=Xp(s)Xv(s)=KqAps(s2ωh2+2ξhωhs+1) (14) 式中:液压固有角频率
ωh=√4βeAp2Vtm (rad/s);液压阻尼比ξh=KceAp√βemVt ;m为活塞及其负载总质量(kg);Kq为流量增益系数;Ap为无杆腔活塞有效面积(m2);Kce为总流量压力系数;Vt为阀腔、液压缸和管道总容积(m3);βe 为有效体积弹性模量(Pa);Xp为液压缸活塞位移量(m)。2.1.4 调平机构数学模型建立
根据式(6)、(7)、(10)可知俯仰、侧倾调平机构中液压缸位移量和平台角度可以看做线性关系。所以调平机构的传递函数为:
G5(s)=θ(s)Xp(s)=Kl1 (15) G6(s)=θ(s)Xp(s)=Kl2 (16) 式中:G5(s)、G6(s)分别为俯仰、侧倾调平机构的传递函数,Kl1、Kl2分别为俯仰、侧倾部分液压缸位移角度系数。
2.2 系统稳定性分析
由经典控制理论[31]可知,要使系统输出稳定,幅值裕度和相位裕度须满足如下条件:
{Kg⩾ (17) 将确定的液压系统元件参数带入俯仰、侧倾系统开环传递函数,令开环增益K = 1,绘制其Bode图。此时,俯仰系统幅值裕度Kg = 39.3 dB,相位裕度γ = 88.9°;侧倾系统Kg = 38.1 dB,γ =88.8°。可见系统不满足稳定性要求。利用公式(18)对开环增益校正,取Kg = 10 dB,则俯仰系统K = 29.2,γ = 54.6°,侧倾系统K = 25.3,γ = 58.4°,此时系统满足稳定性要求。可反推放大器比例系数Ke1、Ke2以设计电路部分。
20\lg \! K = {K_{{\rm{g}}1}} - {K_{\rm{g}}} (18) 式中:Kg1为原系统幅值裕度,Kg为系统期望幅值裕度。
则俯仰系统的开环传递函数:
\begin{aligned} & {G_f}(s) = \frac{{\theta (s)}}{{\Theta (s)}} = \\ & \frac{{29.2}}{{8.4 \! \times \! {{10}^{ - 10}}{s^5} \! + \! 9.68 \times {{10}^{ - 8}}{s^4} \! + \! 2.16 \! \times \! {{10}^{ - 4}}{s^3} \! + \! 2 \! \times \! {{10}^{ - 2}}{s^2} \! + \! s}} \end{aligned} (19) 侧倾系统的开环传递函数:
\begin{aligned} & {G_{\rm{c}}}(s) = \frac{{\theta (s)}}{{\Theta (s)}} =\\ & \frac{{25.3}}{{5.25 \! \times \! {{10}^{ - 9}}{s^5} \! + \! 6.17 \! \times \! {{10}^{ - 7}}{s^4} \! + \! 2.46 \! \times \! {{10}^{ - 4}}{s^3} \! + \! 2.06 \! \times \! {{10}^{ - 2}}{s^2} \! + \! s}} \end{aligned} (20) 2.3 仿真分析
调平系统的控制流程是控制器根据反馈的工作台角度差值计算输出电流控制电液比例阀的开口开度,进而控制液压缸伸缩运动,使平台角度始终维持在0°附近。同时,安装在工作台上的倾角传感器把实时角度发送给控制器,形成闭环反馈控制。控制框图如图4所示。
采用增量式PID控制器,优点是当计算机故障时,系统仍能保持原值,平台不会发生危险情况[32]。计算公式为:
\begin{aligned} u(k) = \; & u(k - 1) + {K_{\rm{p}}}(e(k) - e(k - 1)) + {K_{\rm{i}}}e(k) +\\ & {K_{\rm{d}}}(e(k) - 2e(k - 1) + e(k - 2)) \end{aligned} (21) 式中:u(k)为k时刻控制器输出的电流大小,e(k)为k时刻的控制偏差;Kp、Ki和Kd分别为比例、积分和微分的调节系数。
在Simulink建立调平平台仿真模型,使用零阶保持器,采样时间0.02 s;通过试验法确定俯仰系统比例、积分和微分调节系数分别为0.65、0.04、0,侧倾系统比例、积分和微分调节系数分别为0.85、0.04、0。丘陵山地果园坡度变化平缓,因此干扰选择5°的阶跃信号模拟路面凹坑等突变;因最大工作台俯仰和侧倾调平角度分别为10°、8°,调平角速度0.07 rad/s;确定频率0.1 Hz,幅值为10和8的正弦信号模拟连续坡度变化,此时处于平台极限工作状态。仿真结果如图5、图6所示。
从图5、图6中可以看出:俯仰、侧倾控制系统在阶跃干扰信号下能使工作台很快回到水平位置,调平时间分别为1.6、2.1 s,超调量均为0;俯仰、侧倾控制系统在正弦干扰信号下能使工作台始终保持在0°附近,波动范围分别在0.15°、0.19°内。仿真验证了平台调平的可行性,并且在不同干扰下调平性能较好,工作台角度变化较平稳,满足调平平台设计要求。
3. 安全性仿真实验验证
为测试平台的安全性和工作台调平对倾翻坡度的影响,根据结构和参数在SolidWorks中建立调平平台三维模型,导入Adams并建立倾翻实验台(图7)。实验台以一定角速度转动,平台以纵向、斜向、横向这3种不同姿态,以0、220、440、660、880、1 100 mm不同升降高度和不同载质量情况下进行仿真。当履带所受支持力为0时,实验台倾角δ记为极限倾翻角度(°),仿真结果如图8。
从图8中可以看出:升降高度和载质量一定时,平台在纵向、斜向和横向这3种姿态时的极限倾翻角度逐渐减小;且3种姿态下,升降高度和载质量增加后,平台极限倾翻角度都会减小。有调平时纵向、斜向和横向3姿态下最小倾翻角度相较于无调平时(26.36°、22.35°、20.27°)分别增加了2.47%、19.20%和24.77%,最大倾翻角度相较于无调平时无明显变化。但不同姿态、升降高度和载质量下,平台调平后对其极限倾翻角度的影响又有所不同。比如纵向时,有调平时极限倾翻角度相对于无调平时在分界线以上略有减小,分界线以下略有增加。这是因为剪叉折臂式调平方式会举升工作台,使平台重心沿坡面上移;当升降高度和载质量增加时(超过分界线时),重心位移量在平行坡面方向的投影占比会大于其在垂直坡面方向的占比,故有利于提高平台纵向上的抗倾翻能力。而在斜向和横向姿态时,有调平时极限倾翻角度较无调平时有所增加;当升降高度和载质量两个因素中的一个因素确定时,平台极限倾翻角度增量均随另一因素增加而增大。因为斜向和横向姿态调平时,均需要侧倾调平机构参与其中,使得平台重心在水平面上的投影会靠近上坡方向移动,且平台纵向尺寸大、横向小,重心移动量在横向上的占比大于纵向上占比,从而提升了平台的抗侧倾能力。
总体来说,无调平时最大和最小极限倾翻角度分别为49.85°和20.27°;有调平时最大极限倾翻角度为48.78°,最小为25.29°。整体来讲,最大极限倾翻角度(纵向、升降高度0 mm、载质量0 kg时)降低了2.15%,最小极限倾翻角度(当横向、升降高度1 100 mm、载质量300 kg时)增加了24.77%,平台安全性明显提高。
4. 结 论
(1)根据丘陵山地果园机械化发展现状设计了一款基于剪叉折臂式的自动调平平台,结构简单可靠;通过公式计算结合设计要求验证调平可行性,确定了平台各个部分的尺寸参数、液压缸位移量和平台角度的关系,计算出液压缸所需推力,建立了三维模型。对控制系统数学建模,在Simulink中采用增量式PID控制器对控制系统进行仿真,结果表明此平台有较好的抗阶跃和正弦干扰信号的能力,在极限工作状态下能始终保持在0°附近。
(2)使用Adams对在不同状态、不同举升高度和不同载质量情况下的平台极限倾翻坡度进行仿真。在仿真条件下,果园高位作业平台在调平状态下极限倾翻坡度最小值为25.29°,相较于无调平时增加了24.77%。这说明本文提出的调平机构可有效提高平台的抗倾翻能力。设计的高位调平平台能够满足丘陵山区果园的使用需求。
-
-
[1] Jansson J K, Hofmockel K S. Soil microbiomes and climate change[J]. Nature Reviews Microbiology, 2020, 18(1): 35−46. doi: 10.1038/s41579-019-0265-7
[2] Bardgett R D, van der Putten W H. Belowground biodiversity and ecosystem functioning[J]. Nature, 2014, 515: 505−511. doi: 10.1038/nature13855
[3] Hector A, Bagchi R. Biodiversity and ecosystem multifunctionality[J]. Nature, 2007, 448: 188−190. doi: 10.1038/nature05947
[4] Zhou Z, Wang C, Luo Y. Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality[J]. Nature Communications, 2020, 11(1): 3072. doi: 10.1038/s41467-020-16881-7
[5] Becker J, Eisenhauer N, Scheu S, et al. Increasing antagonistic interactions cause bacterial communities to collapse at high diversity[J]. Ecology Letters, 2012, 15(5): 468−474. doi: 10.1111/j.1461-0248.2012.01759.x
[6] Nielsen U N, Ayres E, Wall D H, et al. Soil biodiversity and carbon cycling: a review and synthesis of studies examining diversity–function relationships[J]. European Journal of Soil Science, 2011, 62(1): 105−116. doi: 10.1111/j.1365-2389.2010.01314.x
[7] Cadotte M W, Jonathan D T, Regetz J, et al. Phylogenetic diversity metrics for ecological communities: integrating species richness, abundance and evolutionary history[J]. Ecology Letters, 2010, 13(1): 96−105. doi: 10.1111/j.1461-0248.2009.01405.x
[8] Wiens J J, Graham C H. Niche conservatism: integrating evolution, ecology, and conservation biology[J]. Annual Review of Ecology, Evolution, and Systematics, 2005, 36(1): 519−539. doi: 10.1146/annurev.ecolsys.36.102803.095431
[9] Goberna M, Verdú M. Phylogenetic-scale disparities in the soil microbial diversity-ecosystem functioning relationship[J]. The ISME Journal, 2018, 12(9): 2152−2162. doi: 10.1038/s41396-018-0162-5
[10] Lee H, Romero J. Climate change 2023: synthesis report[R]// Contribution of working groups Ⅰ, Ⅱ and Ⅲ to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva: IPCC, 2023: 35−115.
[11] Barnard S, van Goethem M W, de Scally S Z, et al. Increased temperatures alter viable microbial biomass, ammonia oxidizing bacteria and extracellular enzymatic activities in Antarctic soils[J]. FEMS Microbiology Ecology, 2020, 96(5): fiaa065. doi: 10.1093/femsec/fiaa065
[12] Pold G, Billings A F, Blanchard J L, et al. Long-term warming alters carbohydrate degradation potential in temperate forest soils[J]. Applied and Environmental Microbiology, 2016, 82(22): 6518–6530.
[13] Pietikäinen, Pettersson M, Bååth E. Comparison of temperature effects on soil respiration and bacterial and fungal growth rates[J]. FEMS Microbiology Ecology, 2005, 52(1): 49−58. doi: 10.1016/j.femsec.2004.10.002
[14] Huang W, Hall S J. Elevated moisture stimulates carbon loss from mineral soils by releasing protected organic matter[J]. Nature Communications, 2017, 8(1): 1774. doi: 10.1038/s41467-017-01998-z
[15] Robinson D A, Campbell C S, Hopmans J W, et al. Soil moisture measurement for ecological and hydrological watershed-scale observatories: a review[J]. Vadose Zone Journal, 2008, 7(1): 358−389. doi: 10.2136/vzj2007.0143
[16] Manzoni S, Schimel J P, Porporato A. Responses of soil microbial communities to water stress: results from a meta-analysis[J]. Ecology, 2012, 93(4): 930−938. doi: 10.1890/11-0026.1
[17] Rousk J, Bååth E, Brookes P C, et al. Soil bacterial and fungal communities across a pH gradient in an arable soil[J]. The ISME Journal, 2010, 4(10): 1340−1351. doi: 10.1038/ismej.2010.58
[18] Waldrop M P, Holloway J M, Smith D B, et al. The interacting roles of climate, soils, and plant production on soil microbial communities at a continental scale[J]. Ecology, 2017, 98(7): 1957−1967. doi: 10.1002/ecy.1883
[19] van der Heijden M G A, Bardgett R D, van Straalen N M. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems[J]. Ecology Letters, 2008, 11(3): 296−310. doi: 10.1111/j.1461-0248.2007.01139.x
[20] Eisenhauer N, Lanoue A, Strecker T, et al. Root biomass and exudates link plant diversity with soil bacterial and fungal biomass[J]. Scientific Reports, 2017, 7(1): 44641. doi: 10.1038/srep44641
[21] Wan X, Chen X, Huang Z, et al. Contribution of root traits to variations in soil microbial biomass and community composition[J]. Plant and Soil, 2021, 460(1−2): 483−495. Wan X, Chen X, Huang Z, et al. Contribution of root traits to variations in soil microbial biomass and community composition[J]. Plant and Soil, 2021, 460(1−2): 483−495.
[22] 陆雅海, 傅声雷, 褚海燕, 等. 全球变化背景下的土壤生物学研究进展[J]. 中国科学基金, 2015, 29(1): 19−24. Lu Y H, Fu S L, Chu H Y, et al. Recent advances in global change and soil biology[J]. Bulletin of National Science Foundation of China, 2015, 29(1): 19−24.
[23] Zogg G P, Zak D R, Ringelberg D B, et al. Compositional and functional shifts in microbial communities due to soil warming[J]. Soil Science Society of America Journal, 1997, 61(2): 475−481. doi: 10.2136/sssaj1997.03615995006100020015x
[24] Chen J, Luo Y, Xia J, et al. Stronger warming effects on microbial abundances in colder regions[J]. Scientific Reports, 2015, 5(1): 18032. doi: 10.1038/srep18032
[25] de Angelis K M, Pold G, Topçuoğlu B D, et al. Long-term forest soil warming alters microbial communities in temperate forest soils[J]. Frontiers in Microbiology, 2015, 6: 00104.
[26] Schindlbacher A, Rodler A, Kuffner M, et al. Experimental warming effects on the microbial community of a temperate mountain forest soil[J]. Soil Biology and Biochemistry, 2011, 43(7): 1417−1425. doi: 10.1016/j.soilbio.2011.03.005
[27] Crowther T W, van den Hoogen J, Wan J, et al. The global soil community and its influence on biogeochemistry[J]. Science, 2019, 365: eaav0550. doi: 10.1126/science.aav0550
[28] Pärn J, Verhoeven J T A, Butterbach-Bahl K, et al. Nitrogen-rich organic soils under warm well-drained conditions are global nitrous oxide emission hotspots[J]. Nature Communications, 2018, 9(1): 1135. doi: 10.1038/s41467-018-03540-1
[29] Hayden H L, Mele P M, Bougoure D S, et al. Changes in the microbial community structure of bacteria, archaea and fungi in response to elevated CO2 and warming in an Australian native grassland soil[J]. Environmental Microbiology, 2012, 14(12): 3081−3096. doi: 10.1111/j.1462-2920.2012.02855.x
[30] Zhang Y, Zhang N, Yin J, et al. Simulated warming enhances the responses of microbial N transformations to reactive N input in a Tibetan alpine meadow[J]. Environment International, 2020, 141: 105795. doi: 10.1016/j.envint.2020.105795
[31] Domeignoz-Horta L A, Pold G, Liu X J A, et al. Microbial diversity drives carbon use efficiency in a model soil[J]. Nature Communications, 2020, 11(1): 3684. doi: 10.1038/s41467-020-17502-z
[32] Zhou Y, Sun B, Xie B, et al. Warming reshaped the microbial hierarchical interactions[J]. Global Change Biology, 2021, 27(24): 6331−6347. doi: 10.1111/gcb.15891
[33] Chen Y, Han M, Yuan X, et al. Warming has a minor effect on surface soil organic carbon in alpine meadow ecosystems on the Qinghai-Tibetan Plateau[J]. Global Change Biology, 2022, 28(4): 1618−1629. doi: 10.1111/gcb.15984
[34] Shen M, Piao S, Chen X, et al. Strong impacts of daily minimum temperature on the green-up date and summer greenness of the Tibetan Plateau[J]. Global Change Biology, 2016, 22(9): 3057−3066. doi: 10.1111/gcb.13301
[35] Yu C, Han F, Fu G. Effects of 7 years experimental warming on soil bacterial and fungal community structure in the Northern Tibet alpine meadow at three elevations[J]. Science of The Total Environment, 2019, 655: 814−822. doi: 10.1016/j.scitotenv.2018.11.309
[36] Xu M, Li X, Kuyper T W, et al. High microbial diversity stabilizes the responses of soil organic carbon decomposition to warming in the subsoil on the Tibetan Plateau[J]. Global Change Biology, 2021, 27(10): 2061−2075. doi: 10.1111/gcb.15553
[37] Wei X, Shi Y, Qin F, et al. Effects of experimental warming, precipitation increase and their interaction on AM fungal community in an alpine grassland of the Qinghai-Tibetan Plateau[J]. European Journal of Soil Biology, 2021, 102: 103272. doi: 10.1016/j.ejsobi.2020.103272
[38] Jiao S, Chen W, Wang J, et al. Soil microbiomes with distinct assemblies through vertical soil profiles drive the cycling of multiple nutrients in reforested ecosystems[J]. Microbiome, 2018, 6(1): 146. doi: 10.1186/s40168-018-0526-0
[39] Edgar R C. UPARSE: highly accurate OTU sequences from microbial amplicon reads[J]. Nature Methods, 2013, 10(10): 996−998. doi: 10.1038/nmeth.2604
[40] Wemheuer F, Taylor J A, Daniel R, et al. Tax4Fun2: prediction of habitat-specific functional profiles and functional redundancy based on 16S rRNA gene sequences[J]. Environmental Microbiome, 2020, 15(1): 11. doi: 10.1186/s40793-020-00358-7
[41] Nguyen N H, Song Z, Bates S T, et al. FUNGuild: an open annotation tool for parsing fungal community datasets by ecological guild[J]. Fungal Ecology, 2016, 20: 241−248. doi: 10.1016/j.funeco.2015.06.006
[42] McMurdie P J, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data[J]. PLoS One, 2013, 8(4): e61217. doi: 10.1371/journal.pone.0061217
[43] Dixon P. VEGAN, a package of R functions for community ecology[J]. Journal of Vegetation Science, 2003, 14(6): 927−930. doi: 10.1111/j.1654-1103.2003.tb02228.x
[44] Zeng Z, Piao S, Li L Z X, et al. Climate mitigation from vegetation biophysical feedbacks during the past three decades[J]. Nature Climate Change, 2017, 7(6): 432−436. doi: 10.1038/nclimate3299
[45] Pisani O, Frey S D, Simpson A J, et al. Soil warming and nitrogen deposition alter soil organic matter composition at the molecular-level[J]. Biogeochemistry, 2015, 123(3): 391−409. doi: 10.1007/s10533-015-0073-8
[46] Ganjurjav H, Gao Q, Zhang W, et al. Effects of warming on CO2 fluxes in an alpine meadow ecosystem on the central Qinghai-Tibetan Plateau[J]. PLoS One, 2015, 10(7): e0132044. doi: 10.1371/journal.pone.0132044
[47] Tong Y, Long Y, Yang Z. Effects of warming and isolation from precipitation on the soil carbon, nitrogen, and phosphorus, and their stoichiometries in an alpine meadow in the Qinghai-Tibet Plateau: a greenhouse warming study[J]. Frontiers in Ecology and Evolution, 2023, 11: 1149240. doi: 10.3389/fevo.2023.1149240
[48] Rui Y, Wang Y, Chen C, et al. Warming and grazing increase mineralization of organic P in an alpine meadow ecosystem of Qinghai-Tibet Plateau, China[J]. Plant and Soil, 2012, 357: 73−87. doi: 10.1007/s11104-012-1132-8
[49] Schmidt I K, Tietema A, Williams D, et al. Soil solution chemistry and element fluxes in three European heathlands and their responses to warming and drought[J]. Ecosystems, 2004, 7(6).
[50] Ma F F, Yan Y, Svenning J C, et al. Opposing effects of warming on the stability of above- and belowground productivity in facing an extreme drought event[J]. Ecology, 2024, 105(1): e4193. doi: 10.1002/ecy.4193
[51] Zhang Y, Zhang N, Yin J, et al. Combination of warming and N inputs increases the temperature sensitivity of soil N2O emission in a Tibetan alpine meadow[J]. Science of the Total Environment, 2020, 704: 135450. doi: 10.1016/j.scitotenv.2019.135450
[52] Yan Y, Wang J, Tian D, et al. Heterotrophic respiration and its proportion to total soil respiration decrease with warming but increase with clipping[J]. Catena, 2022, 215: 106321. doi: 10.1016/j.catena.2022.106321
[53] Bai X, Huang Y, Ren W, et al. Responses of soil carbon sequestration to climate-smart agriculture practices: a meta-analysis[J]. Global Change Biology, 2019, 25(8): 2591−2606. doi: 10.1111/gcb.14658
[54] Zhou J, Li X, Peng F, et al. Mobilization of soil phosphate after 8 years of warming is linked to plant phosphorus-acquisition strategies in an alpine meadow on the Qinghai-Tibetan Plateau[J]. Global Change Biology, 2021, 27(24): 6578−6591. doi: 10.1111/gcb.15914
[55] Zhao J, Xie X, Jiang Y, et al. Effects of simulated warming on soil microbial community diversity and composition across diverse ecosystems[J]. Science of the Total Environment, 2024, 911: 168793. doi: 10.1016/j.scitotenv.2023.168793
[56] Castro H F, Classen A T, Austin E E, et al. Soil microbial community responses to multiple experimental climate change drivers[J]. Applied and Environmental Microbiology, 2010, 76(4): 999−1007. doi: 10.1128/AEM.02874-09
[57] Allison S D, Martiny J B H. Resistance, resilience, and redundancy in microbial communities[J]. Proceedings of the National Academy of Sciences, 2008, 105: 11512−11519. doi: 10.1073/pnas.0801925105
[58] Wu J, Xiong J, Hu C, et al. Temperature sensitivity of soil bacterial community along contrasting warming gradient[J]. Applied Soil Ecology, 2015, 94: 40−48. doi: 10.1016/j.apsoil.2015.04.018
[59] Sui X, Zhang R, Frey B, et al. Land use change effects on diversity of soil bacterial, Acidobacterial and fungal communities in wetlands of the Sanjiang Plain, northeastern China[J]. Scientific Reports, 2019, 9(1): 18535. doi: 10.1038/s41598-019-55063-4
[60] Johnson R J, Stenvinkel P, Andrews P, et al. Fructose metabolism as a common evolutionary pathway of survival associated with climate change, food shortage and droughts[J]. Journal of Internal Medicine, 2020, 287(3): 252−262. doi: 10.1111/joim.12993
[61] Wan P, Zhang F, Zhang K, et al. Soil warming decreases carbon availability and reduces metabolic functions of bacteria[J]. Catena, 2023, 223: 106913. doi: 10.1016/j.catena.2023.106913
[62] Cai P, Sun X, Wu Y, et al. Soil biofilms: microbial interactions, challenges, and advanced techniques for ex-situ characterization[J]. Soil Ecology Letters, 2019, 1(3−4): 85−93. doi: 10.1007/s42832-019-0017-7
[63] Schimel J, Schaeffer S M. Microbial control over carbon cycling in soil[J]. Frontiers in Microbiology, 2012, 3: 348.
[64] Zheng M M, Wang C, Li W X, et al. Soil nutrients drive function and composition of phoC-harboring bacterial community in acidic soils of southern China[J]. Frontiers in Microbiology, 2019, 10: 2654. doi: 10.3389/fmicb.2019.02654
[65] Sandhya V, Ali Sk Z. The production of exopolysaccharide by Pseudomonas putida GAP-P45 under various abiotic stress conditions and its role in soil aggregation[J]. Microbiology, 2015, 84(4): 512−519. doi: 10.1134/S0026261715040153
[66] Wingender J, Neu T R, Flemming H C. Microbial extracellular polymeric substances[M]. Berlin: Springer Berlin Heidelberg, 1999.
[67] Lehmann A, Zheng W, Rillig M C. Soil biota contributions to soil aggregation[J]. Nature Ecology & Evolution, 2017, 1(12): 1828−1835.
[68] Flemming H C, Wingender J. The biofilm matrix[J]. Nature Reviews Microbiology, 2010, 8(9): 623−633. doi: 10.1038/nrmicro2415
[69] Saha I, Datta S, Biswas D. Exploring the role of bacterial extracellular polymeric substances for sustainable development in agriculture[J]. Current Microbiology, 2020, 77(11): 3224−3239. doi: 10.1007/s00284-020-02169-y
[70] Burns R G, de Forest J L, Marxsen J, et al. Soil enzymes in a changing environment: current knowledge and future directions[J]. Soil Biology and Biochemistry, 2013, 58: 216−234. doi: 10.1016/j.soilbio.2012.11.009
[71] Schimel J P, Bennett J. Nitrogen mineralization: challenges of a changing paradigm[J]. Ecology, 2004, 85(3): 591−602. doi: 10.1890/03-8002
[72] Li D, Meng M, Ren B, et al. Different responses of soil fungal and bacterial communities to nitrogen addition in a forest grassland ecotone[J]. Frontiers in Microbiology, 2023, 14: 1211768. doi: 10.3389/fmicb.2023.1211768
-
期刊类型引用(11)
1. 杨涛,孙付春,黄波,吴柏强,冉光泽. 果园作业平台关键技术研究进展. 中国农机化学报. 2024(01): 152-159 .  百度学术
百度学术
2. 汪若尘,蒋亦勇,丁仁凯,孙泽宇,徐可. 基于BP神经网络PID的机电式作业机全向调平控制. 农业工程学报. 2024(23): 52-62 .  百度学术
百度学术
3. 姜欢龙,严重勇,李青涛,梁丽,李佳阳,谭芸颖. 自走式农业机械静态稳定性研究现状及展望. 西华大学学报(自然科学版). 2023(01): 32-41 .  百度学术
百度学术
4. 聂昭成,罗红品,刘威,李光林. 丘陵山地作物信息采集全向自平衡装置的设计与试验. 西南大学学报(自然科学版). 2023(10): 129-138 .  百度学术
百度学术
5. 蒋俞,孙泽宇,汪若尘,夏长高,叶青,郭逸凡. 丘陵山区履带式作业机全向调平系统设计与性能试验. 农业工程学报. 2023(18): 64-73 .  百度学术
百度学术
6. 孙泽宇,夏长高,蒋俞,郭逸凡,汪若尘. 基于QBP-PID的履带式作业机全向调平控制研究. 农业机械学报. 2023(12): 397-406 .  百度学术
百度学术
7. 姜彪,付友,郭靖,张海林,马新华. 煤矿抢险移动式自动调平搭载平台控制系统. 煤矿机械. 2022(04): 181-185 .  百度学术
百度学术
8. 张翠英,仪垂良,刘学峰,任冬梅,张成保. 基于CAN总线的悬浮式转向驱动桥电气控制系统设计. 农业装备与车辆工程. 2021(05): 1-5 .  百度学术
百度学术
9. 缪友谊,陈小兵,朱继平,袁栋,陈伟,丁艳. 果园作业平台研究进展分析. 中国农机化学报. 2021(06): 41-49 .  百度学术
百度学术
10. 李林林,邓干然,林卫国,崔振德,何冯光,李国杰. 农业机械自动调平技术发展现状与趋势. 现代农业装备. 2021(05): 2-7+35 .  百度学术
百度学术
11. 吕昊暾,胡召田,于泳超,康峰,郑永军. 果园高位作业平台自动调平前馈PID控制方法. 农业工程学报. 2021(18): 20-28 .  百度学术
百度学术
其他类型引用(5)



 下载:
下载: