Screening of a highly pathogenic strain against hazelnut weevil and microscopic observation on its infection process
-
摘要: 榛实象甲是目前我国榛园中导致产量损失的首要害虫,生产中以化学防治为主,易导致果品化学残留超标问题。本研究的目的在于筛选榛实象甲的高毒力菌株,以期为其生物学防治提供科学依据。本研究使用2株绿僵菌(CGMCC No.3.7986、3.4607)、1株白僵菌(CGMCC No. 12108)接种榛实象甲成虫,检测其侵染榛实象甲累积死亡率随时间的变化,并用解剖镜与扫描电镜观察了白僵菌侵染榛实象甲成虫的过程。结果表明,白僵菌菌株12108侵染榛实象甲后,在处理后的第4天与第6天累积死亡率分别达到91.67%和100%,远高于同期绿僵菌3.7986的6.67%和17.5%及绿僵菌3.4607的10.0%和22.5%;白僵菌菌株12108的LT50和LT90值分别为2.56和4.42d,远小于绿僵菌3.7986的11.40和17.70d及绿僵菌3.4607的8.80和12.80d;在侵染开始时,白僵菌菌株12108一般从口器、触角、胸足基部、胸足关节等有缝隙的部位开始生长,接种后10d时,该虫表面被浓密的菌丝所覆盖,胸腔内部也充满菌丝,体表开始形成大量分生孢子。以上研究结果确认白僵菌菌株12108对榛实象甲有强致病力,这为榛实象甲生物农药的开发提供了重要依据。Abstract: Hazelnut weevil is the primary pest causing yield loss of hazelnut orchard in China. In practice, chemical spray is the major method of controlling hazelnut weevil. However, frequent chemical spray may cause excessive residual of hazelnut products. The aim of present study is to screen a highly pathogenic strain against nut weevil and provide scientific evidence for its biocontrol. Metarhizium anisopliae var. acridum 3.7986, M. anisopliae 3.4607 and Beauveria bassiana subsp. palomenae (CGMCC No. 12108) were used to infect nut weevil adults, and the time course of accumulated lethal ratio of nut weevil adults was analyzed after inoculation. It was shown that, after inoculated with B. bassiana 12108, the accumulated lethal ratio of nut weevil adults reached 91.67% and 100% on the fourth and sixth day, respectively, which was much higher than in inoculated with M. anisopliae 3.4607 (6.67% and 17.5%), and M. anisopliae 3.4607 (10.0% and 22.5%). LT50 and LT90 of B.bassiana 12108 were 2.56 and 4.42 days, respectively, which were much shorter than that of M. anisopliae 3.7986 (11.40 and 17.70 days) and M. anisopliae 3.4607 (8.80 and 12.80 days). Moreover, the process of B. bassiana infecting nut weevil adults was observed using dissecting microscope and scanning electronic microscope. We found that, at the early stage of infection, B. bassiana 12108 began to grow from joint of mouthparts, antennae, legs and joint base of chest foot. On the 10th day after inoculation, the hazelnut weevil was covered by dense hyphae, and its chest cavity was filled with dense hyphae as well. Simultaneously, a large amount of spores were formed on the body surface.Taken together, our results indicate that B. bassiana 12108 is highly virulence against hazelnut weevil, and provides scientific evidence for further developing of biological pesticide controlling hazelnut weevil.
-
Keywords:
- hazelnut weevil /
- Beauveria bassiana /
- Metarhizium anisopliae /
- biocontrol
-
榛子(Corylus spp.)是壳斗目(Fagales)桦木科(Betulaceae)榛属(Corylus)植物[1],有“坚果之王”的美誉,栽培价值很高,因地制宜的发展榛子产业对于山区经济发展有重要意义[2-3]。东北是我国榛子的传统主产区,随着我国退耕还林政策的实施,榛子被确定为优选树种。近年来,全国各地榛子的人工栽培面积有了迅猛的发展,产业对区域经济的支撑作用日益凸现。目前,榛子因虫害导致产量损失问题、因化学药剂频繁使用导致的食品安全问题日趋严重,严重阻碍了榛子产业的发展。可见,针对榛子的主要害虫研发有效生物农药对该产业的可持续发展有重要意义。
榛实象甲(Curculio nucum)是一种中等大小的甲虫,具有鞘翅目(Coleoptera)象虫科(Curculionidae)的典型特征。在土耳其、意大利等榛子主产国,榛实象甲是榛园中广泛分布的首要害虫,成虫以叶片、嫩芽为食,交配产卵于榛子幼果,幼虫孵化后以榛仁为食,1对成虫可导致约170枚果实的损失[4-6]。在生产中,与其他害虫相比,榛实象甲危害导致的产量损失最多,针对该害虫进行的化学防治最为频繁,易导致果品化学残留超标问题。白僵菌(Beauveria bassiana)与绿僵菌(Metarhizium anisopliae)均是寄生性真菌,可在世界各地的土壤中自然生长,能够用于不少害虫的生物防治,包括:锥蝽(Triatoma infestans)[7]、棕榈象甲(Rhynchophorus ferrugineus)[8]、马铃薯象甲(Leptinotarsa decemlineata)[9]、柑橘灰象甲(Sympiezomias citri)[10]、茶丽纹象甲(Myllocerinus aurolineatus)[11]等。白僵菌与绿僵菌对人畜无害的特点已被人们所熟知,但目前还缺乏针对榛园中榛实象甲的高毒力菌株。因此,本研究的目的是筛选榛实象甲的高毒力菌株,以期为其生物学防治提供科学依据。
1. 材料与方法
1.1 供试材料
供试虫源:供试榛实象甲成虫取自吉林省四平市伊通满族自治县吉林省德胜农牧科技有限公司所属榛园。在6月上旬至7月中旬,于晴天早上05:00—06:30,于4~5年生榛树下平铺2m2的白色塑料布,摇晃枝条,拾取因假死习性掉落至塑料布的榛实象甲成虫。成虫放置于培养皿中,每皿放3头,培养皿放置新鲜榛子叶片3片,然后置于25℃,湿度60%的培养箱中培养备用,培养箱的光照强度10000Lx,光周期为12h光照/12h黑暗,每天更换新鲜榛子叶片1次。
供试菌株:供试菌株有绿僵菌2株(M. anisopliae var. acridum 3.7986; M. anisopliae 3.4607),白僵菌1株(B. bassiana subsp. palomenae (CGMCC No. 12108)),以上菌株均来自中国普通微生物菌种保藏管理中心,其中,白僵菌菌株为本实验室专利保藏。上述3个菌株转接至SDY液体培养基中进行活化培养,培养温度25℃,转速100r/min,培养1周后,转接至马铃薯葡萄糖琼脂培养基PDA中继续培养10d。待孢子形成后,收集孢子,通过孢子萌发实验,确认孢子萌发率在95%以上,随后用无菌水将孢子稀释至1×107个/mL,并添加0.3%的吐温80备用。
1.2 研究方法
1.2.1 不同菌株对榛实甲的致病力检测
软镊取榛实象甲成虫,浸泡在100mL制备好的3个菌株的悬液中8s,以无菌水加0.3%的吐温80作为对照,无菌滤纸吸附多余液体。取新鲜榛子叶片置于直径9cm的培养皿中,每皿放置3片叶,接种后的榛实象甲虫转入培养皿中,每皿放3头,培养方法同上。对照处理60头,白僵菌和绿僵菌每个菌株侵染榛象甲成虫180头,每天检查、统计成虫死亡数目,计算累积死亡率并用体视镜进行拍照。在MICROSOFT OFFICE EXCEL ®2007中,用多项式对3个菌株的累积死亡率曲线变化趋势进行模拟,利用拟合程度最佳的方程计算LT50和LT90值。
1.2.2 电镜样品的制备
榛实象甲成虫样品用1%OsO4固定12h,去离子水清洗3次,每次20min。50%、70%、90%乙醇梯度脱水,每级20min;100%乙醇3次,每次30min。脱水后样品用等比例乙醇和乙酸异戊酯处理30min,随后用纯乙酸异戊酯浸泡处理3.0h,K850型临界点干燥仪进行干燥(Quorum Technologies, Ashford, UK)。为防止成虫表面的菌丝及分生孢子在电子束轰击下脱落污染电镜的真空样品室,离子喷镀前用洗耳球吹拂虫体表面数次,然后用IS12/IS13型离子溅射镀膜仪镀金,用KYKY-1000B型扫描电镜观察白僵菌在虫体表面的生长情况[12]。
2. 结果与分析
2.1 不同菌株侵染榛实象甲累积死亡率变化
3个菌株侵染榛实象甲后,累积死亡率存在明显的差异(图 1)。与对照及2株绿僵菌相比,白僵菌菌株12108侵染榛实象甲后,累积死亡率曲线呈迅速上升趋势,在处理后的第4天达到91.67%,第6天即达到100%,远高于同期绿僵菌3.7986的6.67%和17.5%及绿僵菌3.4607的10.0%和22.5%。在处理后的第14天,绿僵菌3.7986和3.4607的致死率分别为62.5%和95.0%。可见,绿僵菌3.7986和3.4607对榛实象甲成虫均有一定的杀灭效果,但与白僵菌菌株12108相比较,供试的2株绿僵菌侵染周期较长,累积死亡率也低。
![]() 图 1 接种不同绿僵菌与白僵菌菌株的榛实象甲累积死亡率的变化曲线CK表示对照,3.7986表示绿僵菌菌株3.7986,3.4607表示绿僵菌菌株3.4607,12108表示白僵菌菌株12108。下同。Figure 1. Cumulative mortality of nut weevil adult after inoculation with differing strains of M. anisopliae and B. bassianaCK means control, 3.7986 means M. anisopliae var. acridum 3.7986, 3.4607 means M. anisopliae 3.4607, 12108 means B. bassiana subsp. palomenae 12108 (CGMCC No. 12108). The same below.
图 1 接种不同绿僵菌与白僵菌菌株的榛实象甲累积死亡率的变化曲线CK表示对照,3.7986表示绿僵菌菌株3.7986,3.4607表示绿僵菌菌株3.4607,12108表示白僵菌菌株12108。下同。Figure 1. Cumulative mortality of nut weevil adult after inoculation with differing strains of M. anisopliae and B. bassianaCK means control, 3.7986 means M. anisopliae var. acridum 3.7986, 3.4607 means M. anisopliae 3.4607, 12108 means B. bassiana subsp. palomenae 12108 (CGMCC No. 12108). The same below.2.2 不同菌株的LT50和LT90值的差异
在MICROSOFT OFFICE EXCEL ®2007中,用多项式对3个菌株的累积死亡率曲线变化趋势进行模拟,获取拟合程度最佳(相关系数最高)的模拟方程。以模拟方程为依据,对LT50和LT90值进行了计算,结果如表 1所示。白僵菌菌株12108的LT50和LT90值分别为2.56和4.42d,远小于绿僵菌3.7986的11.40和17.70d及绿僵菌3.4607的8.80和12.80d,这与图 3的结果相一致。可见,白僵菌菌株12108对榛实象甲具有强致病力。
表 1 不同菌株侵染榛实象的累积死亡率模拟方程及LT50、LT90值Table 1. Simulation equation and LT50 and LT90 values of different fungal strains infecting nut weevil菌株编号
Strain code累积死亡率模拟方程
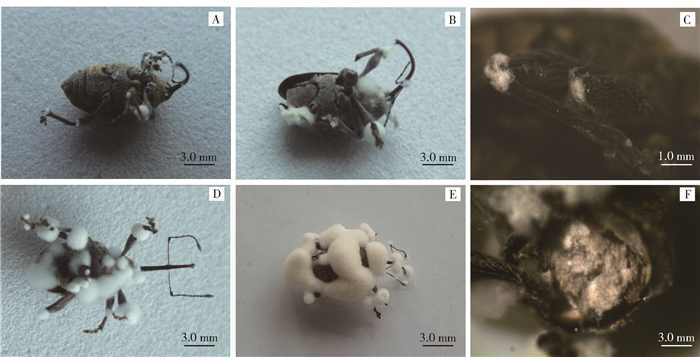
Simulating equation of cumulative mortalityR2 LT50/d LT90/d 3.7986 y=0.0647x2+4.4731x-9.4277 0.9833 11.40 17.70 3.4607 y=0.2122x2+5.1614x-11.783 0.9786 8.80 12.80 12108 y=-3.8839x2+48.688x-49.417 0.9805 2.56 4.42 注:累积死亡率模拟方程中x为接种后天数,y为累积死亡率。Notes: x and y in simulating equations represent days after inoculation and cumulative mortality, respectively. ![]() 图 3 体视镜观察白僵菌侵染榛实象甲成虫过程A.接种后第4天的成虫;B.接种后第6天的成虫;C.接种后第6天的胸足形成白色菌丝;D.接种后第8天的成虫;E.接种后第10天的成虫;F.接种后10天的成虫胸腔中形成大量白色菌丝。Figure 3. Process of B. bassiana infecting nut weevil adults using stereomicroscopeA, adult, 4 days after inoculation; B, adult, 6 days after inoculation; C, white hyphae formed on the pereiopoda; D, adult, 8 days after inoculation; E, adult, 10 days after inoculation; F, large amount of white hyphae formed in the chest cavity.
图 3 体视镜观察白僵菌侵染榛实象甲成虫过程A.接种后第4天的成虫;B.接种后第6天的成虫;C.接种后第6天的胸足形成白色菌丝;D.接种后第8天的成虫;E.接种后第10天的成虫;F.接种后10天的成虫胸腔中形成大量白色菌丝。Figure 3. Process of B. bassiana infecting nut weevil adults using stereomicroscopeA, adult, 4 days after inoculation; B, adult, 6 days after inoculation; C, white hyphae formed on the pereiopoda; D, adult, 8 days after inoculation; E, adult, 10 days after inoculation; F, large amount of white hyphae formed in the chest cavity.2.3 白僵菌菌落、菌丝特征及侵染榛实象甲过程观察
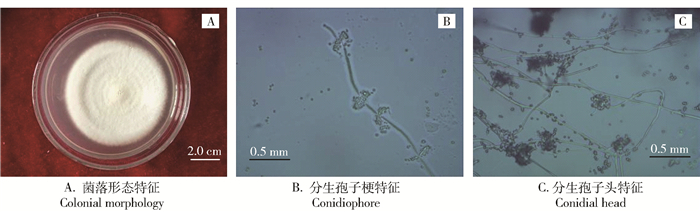
白僵菌菌株12108在PDA培养基上的形态特点如图 2A所示。菌落色泽为乳白色,菌落质地初期绒毛状,培养后期可见乳粉状的孢子层,菌落薄、中间褶皱、呈放射状生长。分生孢子梗着生在营养菌丝上,产孢细胞浓密簇生于菌丝或泡囊上,产孢细胞基部多为球形,近球形,产孢轴较长,轴上具小齿突,呈膝状弯曲(“之”字形弯曲),基上着生分生孢子(图 2B)。至培养后期,在分生孢子梗或菌丝上聚成球状至卵状的相当密实的孢子头(图 2C)。分生孢子单孢,透明,薄壁,无厚垣孢子。
白僵菌菌株12108接种榛实象甲后的第2天,榛实象甲开始出现死亡,此时,刚死亡的成虫表面肉眼尚不能看见明显的菌丝生长。在接种后的第4天,死亡成虫的口器、触角、胸足基部、胸足关节等部位开始出现明显的白色菌丝生长(图 3A)。在接种后的第6天,除上述部位以外,成虫腹部也开始出现明显的白色菌丝生长(图 3B),菌丝生长量逐渐变大;由图 3C可见,榛实象甲胸足上白僵菌的生长自关节连接处开始,腿节、胫节、跗节均有白色菌丝的生长,然后向四周漫延。接种第8与第10天时(图 3D、E),成虫背部、胸部、胸足、口器、触角等部位均被浓密的菌丝所覆盖,死亡成虫菌丝生长特征与培养基生长特征相似(图 3E,图 2A)。接种第10天时,用解剖刀切除成虫头部,可观察到胸腔中已被白僵菌的白色菌丝所充满(图 3F)。
2.4 白僵菌侵染榛实象甲过程扫描电镜观察
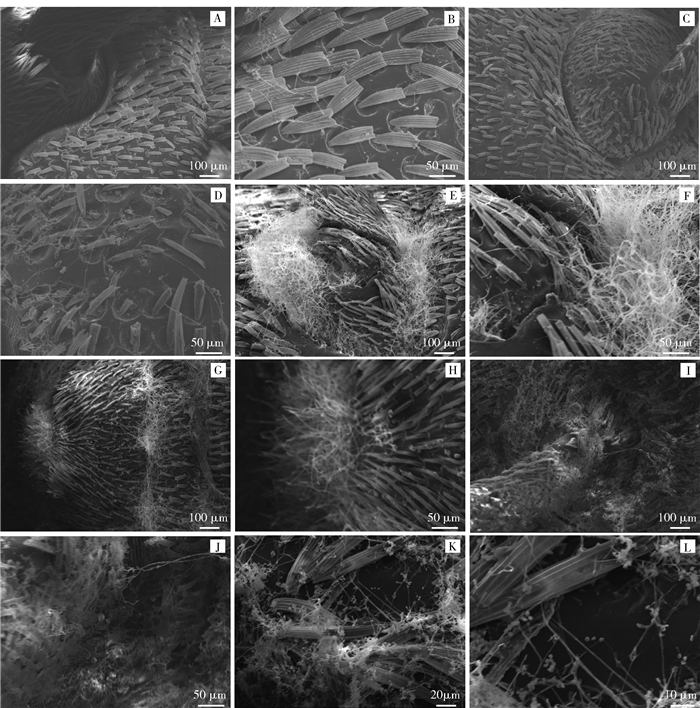
白僵菌菌株12108接种榛实象甲后,口器、触角、胸足关节等部位很容易大量生长(图 3),腹部表面菌丝的生长相对滞后。采用扫描电镜观察了白僵菌侵染榛实象甲腹面的生长情况(图 4)。榛实象甲虫腹部可观察到有大量盾状刚毛,在接种后第4天,胸足的转足附近有零星的菌丝分布(图 4A、B);在接种后第6天,胸足转节、腿节等部位的菌丝进一步生长(图 4C、D);接种后8天,胸足转节附近被丛状菌丝所覆盖,而此时昆虫的尾部,也常可观察到丛状的菌丝(图 4E、F)。接种后10天,胸足附近的菌丝量几乎完全覆盖转节、腿节等部位(图 4I、J);在成虫腹面,接种后10天可观察到有白僵菌的孢子大量形成(图 4K、L)。
![]() 图 4 扫描电镜下白僵菌侵染榛实象甲成虫过程A、B为接种后第4天的成虫,有少量菌丝在腹面形成;C、D为接种后第6天的成虫,有菌丝在腹面形成;E、F为接种后第8天的成虫胸足基部形成大量菌丝;G、H为接种后第8天的成虫尾部形成大量菌丝;I、J为接种后第10天的成虫表面菌丝量持续增长;K、L为接种后第10天的成虫表面开始形成孢子。Figure 4. Process of B. bassiana infecting nut weevil adults using scanning electronic microscopeA and B, a small amount of hyphae formed on the abdomen surface, 4 days after inoculation; C and D, hyphae formed on the abdomen surface, 6 days after inoculation; E and F, large amount of hyphae formed at the base of thoracic leg, 6 days after inoculation; G and H, large amount of hyphae formed at the rear of adult, 8 days after inoculation; I and J, hyphae amount increased continually, 10 days after inoculation; K and L, large amount of spores formed on the body surface, 10 days after inoculation.
图 4 扫描电镜下白僵菌侵染榛实象甲成虫过程A、B为接种后第4天的成虫,有少量菌丝在腹面形成;C、D为接种后第6天的成虫,有菌丝在腹面形成;E、F为接种后第8天的成虫胸足基部形成大量菌丝;G、H为接种后第8天的成虫尾部形成大量菌丝;I、J为接种后第10天的成虫表面菌丝量持续增长;K、L为接种后第10天的成虫表面开始形成孢子。Figure 4. Process of B. bassiana infecting nut weevil adults using scanning electronic microscopeA and B, a small amount of hyphae formed on the abdomen surface, 4 days after inoculation; C and D, hyphae formed on the abdomen surface, 6 days after inoculation; E and F, large amount of hyphae formed at the base of thoracic leg, 6 days after inoculation; G and H, large amount of hyphae formed at the rear of adult, 8 days after inoculation; I and J, hyphae amount increased continually, 10 days after inoculation; K and L, large amount of spores formed on the body surface, 10 days after inoculation.3. 讨论
理想的真菌杀虫剂有如下特点:表皮侵染、环境友好,易于大量生产[13-14]。高毒力的菌株是榛实象甲生物防治的重要基础。总的说来,累积死亡率的大小、半致死时间(LT50)能外映生物菌剂对目标害虫的毒力,累积死亡率越高,半致死时间(LT50)越小,意味着生物菌剂具有更高的毒力。本研究结果表明,3个检测菌株均对榛实象甲有一定的杀灭效果,但毒力差异很大,白僵菌菌株12108侵染榛实象甲后,在处理后的第6天累积死亡率达到100%,而此时绿僵菌3.7986和3.4607的累积死亡率仅为17.5%和22.5%;白僵菌菌株12108的LT50为2.56d,远少于绿僵菌3.7986和绿僵菌3.4607的11.40和8.80d。有研究表明,1.0×108孢子/mL白僵菌菌株Bb2-1孢悬液处理(26℃)柑橘灰象甲成虫后,其LT50值为3.77d[10];1.0×107孢子/mL白僵菌菌株XJBb3005菌株侵染茶丽纹象甲成虫后,LT50值为4.45d[11]。可见,与其他白僵菌菌株相比,白僵菌菌株12108侵染榛实象甲的LT50也明显偏小,这可能意味着该菌株具有超强的致病力,而其毒力的广谱性还有待进一步的研究。
一般而言,真菌在宿主表皮的附着是真菌与宿主建立稳定共生关系的前提条件,如果宿主表皮的营养条件对寄生真菌适宜,孢子能够快速萌发,这将有利于寄生关系的建立[14-17]。此前,用绿僵菌(M. anisopliae var. acridum CoM 02)对榛实象甲的幼虫进行了侵染,用扫描电镜可以看见其孢子在幼虫体表附着、萌发、生长、产孢的全过程,其侵染方式为孢子萌发形成芽管,直接侵入幼虫体表,其LT50为7.05d[3]。在本研究中,榛实象甲的成虫外壳坚硬,表面有大量的刚毛覆盖,白僵菌菌株12108侵染后的成虫并没有观察到孢子在成虫体表直接附着和萌发,而是从口器、触角、胸足基部、胸足关节等有缝隙的部位直接萌发进入体内,其LT50仅为2.56d,这种组织接缝侵入方式似乎远较从体表侵入高效,可绕开坚硬的体表直接从薄弱部位侵入,这可能是本研究中白僵菌菌株12108具有超强毒力的原因,该菌株能否开发形成有效的生物菌剂还有待进一步田间试验的证实。
-
图 1 接种不同绿僵菌与白僵菌菌株的榛实象甲累积死亡率的变化曲线
CK表示对照,3.7986表示绿僵菌菌株3.7986,3.4607表示绿僵菌菌株3.4607,12108表示白僵菌菌株12108。下同。
Figure 1. Cumulative mortality of nut weevil adult after inoculation with differing strains of M. anisopliae and B. bassiana
CK means control, 3.7986 means M. anisopliae var. acridum 3.7986, 3.4607 means M. anisopliae 3.4607, 12108 means B. bassiana subsp. palomenae 12108 (CGMCC No. 12108). The same below.
图 3 体视镜观察白僵菌侵染榛实象甲成虫过程
A.接种后第4天的成虫;B.接种后第6天的成虫;C.接种后第6天的胸足形成白色菌丝;D.接种后第8天的成虫;E.接种后第10天的成虫;F.接种后10天的成虫胸腔中形成大量白色菌丝。
Figure 3. Process of B. bassiana infecting nut weevil adults using stereomicroscope
A, adult, 4 days after inoculation; B, adult, 6 days after inoculation; C, white hyphae formed on the pereiopoda; D, adult, 8 days after inoculation; E, adult, 10 days after inoculation; F, large amount of white hyphae formed in the chest cavity.
图 4 扫描电镜下白僵菌侵染榛实象甲成虫过程
A、B为接种后第4天的成虫,有少量菌丝在腹面形成;C、D为接种后第6天的成虫,有菌丝在腹面形成;E、F为接种后第8天的成虫胸足基部形成大量菌丝;G、H为接种后第8天的成虫尾部形成大量菌丝;I、J为接种后第10天的成虫表面菌丝量持续增长;K、L为接种后第10天的成虫表面开始形成孢子。
Figure 4. Process of B. bassiana infecting nut weevil adults using scanning electronic microscope
A and B, a small amount of hyphae formed on the abdomen surface, 4 days after inoculation; C and D, hyphae formed on the abdomen surface, 6 days after inoculation; E and F, large amount of hyphae formed at the base of thoracic leg, 6 days after inoculation; G and H, large amount of hyphae formed at the rear of adult, 8 days after inoculation; I and J, hyphae amount increased continually, 10 days after inoculation; K and L, large amount of spores formed on the body surface, 10 days after inoculation.
表 1 不同菌株侵染榛实象的累积死亡率模拟方程及LT50、LT90值
Table 1 Simulation equation and LT50 and LT90 values of different fungal strains infecting nut weevil
菌株编号
Strain code累积死亡率模拟方程
Simulating equation of cumulative mortalityR2 LT50/d LT90/d 3.7986 y=0.0647x2+4.4731x-9.4277 0.9833 11.40 17.70 3.4607 y=0.2122x2+5.1614x-11.783 0.9786 8.80 12.80 12108 y=-3.8839x2+48.688x-49.417 0.9805 2.56 4.42 注:累积死亡率模拟方程中x为接种后天数,y为累积死亡率。Notes: x and y in simulating equations represent days after inoculation and cumulative mortality, respectively. -
[1] CHENG Y Q, LIU J F, ZHANG H D, et al. Transcriptome analysis and gene expression profiling of abortive and developing ovules during fruit development in hazelnut[J/OL]. PLoS One, 2015, 10(4): e0122072[2016-08-03]. DOI: 10.1371/journal.pone.0122072.
[2] 梁维坚, 王贵禧.大果榛子栽培实用技术[M].北京:中国林业出版社, 2015. LIANG W J, WANG G X. Hybrid hazelnut cultivation technology[M]. Beijing: China Forestry Publishing House, 2015.
[3] CHENG Y Q, LIU T, ZHAO Y X, et al. Evaluation of pathogenicity of the fungi Metarhizium anisopliae and Beauveria bassiana in hazelnut weevil (Curculio nucum L., Coleoptera, Curculionidae) larvae[J]. Indian Journal of Microbiology, 2016, 56(4): 405-410. doi: 10.1007/s12088-016-0614-4
[4] CHENG Y Q, WANG J, LIU J F, et al. Analysis of ovary DNA methylation during delayed fertilization in hazel using the methylation-sensitive amplification polymorphism technique[J]. Acta Physiologiae Plantarum, 2015, 37(11): 231. doi: 10.1007/s11738-015-1984-7
[5] AKCA I, TUNCER C. Biological control and morphological studies on nut weevil (Curculio nucum l. col., curculionidae)[J]. Acta Horticculturae, 2005, 686: 413-420. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=0467201ef2f2fe6e9f62d4c394ae2a02
[6] BATALLA-CARRERA L, MORTON A, GARCIA-DEL-PINO F. Virulence of entomopathogenic nematodes and their symbiotic bacteria against the hazelnut weevil Curculio nucum[J]. Journal of Applied Entomology, 2015, 140(1-2): 115-123. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=10.1111/jen.12265
[7] LECUONA R E, EDELSTEIN J D, BERRETTA M F, et al. Evaluation of Beauveria bassiana (hyphomycetes) strains as potential agents for control of Triatoma infestans (Hemiptera: Reduviidae)[J]. Journal of Medical Entomology, 2016, 38(2): 172-179. https://www.ncbi.nlm.nih.gov/pubmed/11296819
[8] BURGESS G. Use of a solid formulation of Beauveria bassiana for biocontrol of the red palm weevil (Rhynchophorus ferrugineus) (Coleoptera: Dryophthoridae) under field conditions in SE Spain[J]. Florida Entomologist, 2015, 94(4): 737-747. https://www.jstor.org/stable/23065823
[9] WRAIGHT S P, RAMOS M E. Delayed efficacy of Beauveria bassiana, foliar spray applications against Colorado potato beetle: impacts of number and timing of applications on larval and next-generation adult populations[J]. Biological Control, 2015, 83: 51-67. doi: 10.1016/j.biocontrol.2014.12.019
[10] 王定锋, 黎健龙, 王庆森, 等.柑橘灰象甲一株高毒力白僵菌菌株的筛选鉴定及培养特性[J].中国生物防治学报, 2014, 30(6):750-758. http://d.old.wanfangdata.com.cn/Periodical/zgswfz201406007 WANG D F, LI J L, WANG Q S, et al. Selection, identification and culture characteristics of a highly virulent strain of Beauveria towards Sympiezomias citri[J]. Chinese Journal of Biological Control, 2014, 30(6): 750-758. http://d.old.wanfangdata.com.cn/Periodical/zgswfz201406007
[11] 王定锋, 刘丰静, 李慧玲, 等.球孢白僵菌XJBb3005对茶丽纹象甲致病力的时间-剂量-死亡率模型分析[J].福建农业学报, 2013, 28(8):807-811. doi: 10.3969/j.issn.1008-0384.2013.08.016 WANG D F, LIU F J, LI H L, et al. Time-dose-mortality model analysis of Beauveria bassiana XJB3005 against Myllocerinus aurolineatus[J]. Fujian Journal of Agricultural Sciences, 2013, 28(8): 807-811. doi: 10.3969/j.issn.1008-0384.2013.08.016
[12] ZHOU X, WANG D W, GUO K, et al. Germination and sporulation of Pandora delphacis (Entomophthoromycota: Entomophthorales) on the rice pest Nilaparvata lugens: SEM observation[J]. Mycosystema, 2014, 33(4): 819-826. http://d.old.wanfangdata.com.cn/Periodical/jwxt201404009
[13] CUI Q, ZHANG Y, ZANG Y, et al. Screening of high toxic Metarhizium strain against Plutella xylostella and its marking with green fluorescent protein[J]. World Journal Microiology Biotechnology, 2014, 30(10): 2767-2773. doi: 10.1007/s11274-014-1700-6
[14] 黄旭, 黄韵姗, 张静宇, 等.昆虫体内不同微生物间互作关系的研究进展[J].中国生物防治学报, 2015, 31(6):936-945. http://d.old.wanfangdata.com.cn/Periodical/zgswfz201506017 HUANG X, HUANG Y S, ZHANG J Y, et al. Interactions of various microbes in insects: a review[J]. Chinese Journal of Biological Control, 2015, 31(6): 936-945. http://d.old.wanfangdata.com.cn/Periodical/zgswfz201506017
[15] WICHADAKUL D, KOBMOO N, INGSRISWANG S, et al. Insights from the genome of ophiocordyceps polyrhachis-furcata to pathogenicity and host specificity in insect fungi[J/OL]. BMC Genomics, 2015, 16(1): 881[2016-07-15]. DOI: 10.1186/s12864-015-2101-4.
[16] ARAUJO J P, HUGHES D P. Diversity of entomopathogenic fungi: which groups conquered the insect body?[J]. Advances in Genetics, 2016, 94: 1-39. doi: 10.1016/bs.adgen.2016.01.001
[17] AGRAWAL Y, NARWANI T, SUBRAMANIAN S. Genome sequence and comparative analysis of clavicipitaceous insect-pathogenic fungus Aschersonia badia with Metarhizium spp.[J]. BMC Genomics, 2016, 17(1): 367. doi: 10.1186/s12864-016-2710-6
-
期刊类型引用(4)
1. 马润平,马玲,苏光祥,申东晨,宋喜梅,戴华,王琪,马晓乾. 球孢白僵菌对榛实象成虫毒力及解毒酶的影响. 东北林业大学学报. 2024(07): 125-129 .  百度学术
百度学术
2. 邓祥,赵志玲,修冬莹,黄赫,王冠群,张凯鹏. 加强榛子果林病虫害可持续防控的建议. 吉林林业科技. 2024(05): 46-48 .  百度学术
百度学术
3. 连俊华,白应统,邱雅林,白帅军,郭芳. 辽东栎种实害虫为害特点及防治措施研究. 种子科技. 2022(06): 68-71 .  百度学术
百度学术
4. 张兴政,李欣宇,程云清,刘剑锋. 榛子中的杀虫剂残留GC-MS检测与膳食风险评估. 南京林业大学学报(自然科学版). 2021(02): 213-219 .  百度学术
百度学术
其他类型引用(5)




 下载:
下载: