Regulatory effect of stem cambium gibberellin on plant growth and development
-
摘要:目的赤霉素(GAs)是一种重要的植物激素,对植物生长发育具有广泛的调控作用。本文通过对茎部形成层赤霉素在植物生长发育中的调控作用进行研究,为阐明植物体内源活性GAs的运输及其调控作用提供参考。方法本研究通过转基因方法特异下调烟草茎部形成层活性GAs含量,探究其对植株茎部、叶片、根生长发育的调控作用;在此基础上,利用35S:: PtGA20ox、35S:: PtGA2ox1转基因烟草株系分别作接穗和砧木进行微嫁接和施加外源GAs,探究内源与外源活性GAs在烟草体内的运输特性。结果结果表明,茎部形成层特异启动子LMX5介导的PtGA2ox1转基因烟草植株茎部GAs含量与野生型相比显著降低,而叶与根中含量无明显变化;通过石蜡切片观察发现茎部形成层GAs含量降低明显延缓了茎部木质部的发育,同时对植物的叶片、侧根的生长也有抑制作用;对不同转基因株系微嫁接分析发现,外源施加的GAs可以恢复茎部形成层GAs缺乏引起的表型,而内源活性GAs却不能自由运输,也不能恢复表型;结论茎部形成层GAs含量不仅对茎部木质部的发育有重要作用,并且对叶片的生长及不定根的发育也有调控作用,利用微嫁接的方法验证了活性GAs在烟草中的运输机制,明确了GAs在调控植物组织发育中的重要意义,为今后GAs在林木中的应用奠定了理论基础。Abstract:Objective Gibberellin (GAs) is an important plant hormone, which has a wide range of regulatory effects on plant growth and development. In this paper, the regulatory role of gibberellin in stem cambium in plant growth and development was studied, providing reference for elucidation of the transport and regulatory role of source active GAs in plants.Method In this study, the content of active GAs in stem cambium was specifically down-regulated by transgenic method to explore its regulatory effect on the growth and development of plant stems, leaves and roots. On this basis, 35S:: PtGA20ox and 35S:: PtGA2ox1 transgenic tobacco strains were used as scions and rootstocks respectively for micrografting and application of external GAs, to explore the transport characteristics of endogenous and exogenous active GAs in tobacco.Result The results showed that the content of GAs in the stem of PtGA2ox1 transgenic plants mediated by the specific promoter LMX5 in the stem cambium was significantly lower than that in the wild type, while there was no significant change in the content of GAs in the leaves and roots. By observing paraffin sections, we found that the decrease of GAs content in stem cambium significantly delayed the development of stem xylem and inhibited the growth of leaf and lateral root. The analysis of micrografting of different transgenic lines shows that the exogenous GAs can restore the phenotype caused by the lack of stem cambium GAs, while the endogenous active GAs cannot be transported freely or restore the phenotype.Conclusion Stem cambium GAs content is not only an important role in the development of stem xylem, and development of the growth of leaves and adventitious roots also have regulation function, the method of using micro grafting reactive GAs is verified in transport mechanism in the tobacco, it has been clear about the GAs in the regulating plant tissue development, for the future GAs thus lay a foundation for the application in the trees.
-
Keywords:
- gibberellin /
- forming layer /
- transgenic /
- growth and development /
- tobacco micrografting
-
赤霉素(GAs)是一种重要植物激素,在植物整个生命周期中对生长发育的各个阶段具有广泛的调控作用[1-2]。GAs通过促进细胞分裂和细胞伸长[3-6]进而促进茎部节间的伸长生长。李哲馨[7]的研究表明GAs在烟草茎部形成层发挥重要作用,对植株生长和木质部发育具有明显的促进作用。因此,GAs代谢调控在速生林林木育种中具有重要的应用潜力;然而,以往的研究都是基于施加外源活性GAs或全株组成型调控GAs含量的分析,并没有考虑活性GAs在活体内的运输特性对研究结果的影响,有研究表明移动的GAs对茎的次生生长与木质部形成层的发育具有直接的促进作用[8]。另外,由于GAs参与调控多个发育过程,特异下调茎部GAs含量在延缓茎部生长的同时,也会抑制不定根的发生[9],影响根尖的径向生长[10]。有研究表明茎部GAs在低温胁迫的响应过程中具有反馈调控作用[11-12],因此,开展对茎部特异性GAs含量的调控研究,为GAs代谢调控植物生长发育在林木育种中的实际应用提供理论基础。
形成层位于木质部和韧皮部之间,是一种重要的分生组织,细胞分裂旺盛,向外分裂形成新的韧皮部细胞,向茎的中轴方向分裂形成新的木质部细胞,大量调控木材合成的基因在此过程中表达[13]。研究发现在形成层细胞分裂时,GAs加快了形成层细胞的分裂速度,促进了细胞的伸长,从而促进导管及筛管的伸长[14]。此后的研究中进一步证实了形成层GAs对植株生长发育有促进作用,但在外源施加GAs时,尚不能排除由于植物组织损伤而造成植株自身激素含量变化的误差,并且植物某一发育时间或某一组织中的外源激素含量的测定也难以精准,给实验带来很多不确定性[15]。因此,特异性降低植物形成层GAs含量对植物生长发育的调控作用有待于进一步深入研究。
已有研究表明,在植物体内自由运输的可能并不是活性GAs,而是其前体GA12[16]。植物嫁接是研究活性物质长距离运输特性的有效手段[17]。本实验通过嫁接不同激素含量的转基因植株及野生型WT植株再配合外施赤霉素进行实验分析,验证活性GAs在烟草中的运输机制,更深入地探讨组织特异性GAs含量对植株生长发育的调控作用,对今后GAs调控植物各组织发育的研究具有重要意义,为林木速生优质新品种定向培育策略的制定提供理论基础。
1. 材料与方法
1.1 实验材料
以本实验室遗传转化获得的烟草转基因株系LMX5:: PtGA2ox1、35S:: PtGA20ox、35S:: PtGA2ox1及野生型烟草为研究材料(其中35S:: PtGA20ox、35S:: PtGA2ox1来自林木遗传育种实验室的保存材料),分别记作L:G2、35:G20、35:G2与WT。PtGA2ox1基因克隆于毛白杨,转基因受体为野生型烟草W38株系(Nicotiana tabacum cultivar Wisconsin 38)。所有植物材料均在不含任何激素的MS培养基上,组培室温度为25 ℃,光照周期为16 h光照/8 h黑暗,将继代培养2月苗龄的生根苗移栽到温室,在30 ℃自然光照下培养。
1.2 烟草形成层赤霉素特异性调控转基因株系的获得
用毛白杨的成熟叶片提取高质量RNA,反转录获得cDNA,再以cDNA为模板克隆赤霉素代谢中的关键基因PtGA2ox1,构建表达载体LMX5::PtGA2ox1,并转化表达载体到农杆菌中。通过农杆菌介导的转基因技术获得以木质部形成层特异性启动子(LMX5)启动的烟草转基因株系L:G2[18]。
1.3 基因表达分析及GAs含量的测定
分别提取转基因烟草L:G2、35:G2及WT植株茎部、叶片和根部组织的总RNA,反转录得到cDNA,根据差异片段序列设计引物,半定量RT-PCR反应条件:95 ℃变性3 min,随后进行以下40个循环:95 ℃变性3 s,60 ℃退火20 s,72 ℃延伸30 s。以Actin为内标基因,扩增引物序列为FW:5′-CACAAGCCAGCACTTCAACAG-3′,RV:5′-ATGCCTTAACCAGGAGGTGC-3′。将每个扩增反应做3次重复实验,每次均设置阴性对照,并利用软件Bio-Rad CFXManager 2.0读取相关数据。
分别取组织培养30 d的35:G20、35:G2、L:G2与WT植株的叶、茎、根3个部位的样品各3 g,将其放在研钵中进行研磨,用LC-MS(液相色谱−质谱联用仪)对样品进行测定分析[19],每个样本进行3次重复实验。
1.4 烟草的微嫁接
以35:G2烟草转基因株系的顶芽作接穗、35:G20植株茎杆的一段作砧木,从砧木的横截面中心向下垂直的劈开切接口,使劈口长度与接穗的削面相吻合,再用锡箔纸将接穗与砧木紧密包裹,得到以35:G2为接穗,35:G20为砧木的独立嫁接苗(以下写作为35:G2//35:G20),以同样方法分别获得嫁接苗35:G20//35:G2、35:G2//WT以及WT//WT。
1.5 植株各部分生长性状的测定
挑选培养45 d的无菌生根苗L:G2、35:G2、35:G20与WT,分别对其茎部进行切片,用0.5%番红染液染色后对木质部进行显微观察并拍照。
将烟草转基因株系L:G2、35:G2和35:G20的顶芽扦插在无激素的MS培养基上,设置WT为对照,培养20 d后对各组的生根情况进行观察,再从4组株系中各选取30株,对其不定根的长度进行测量,计算平均值后与对照组一同进行t检验。
2. 结果与分析
2.1 转基因株系的获得及基因表达分析
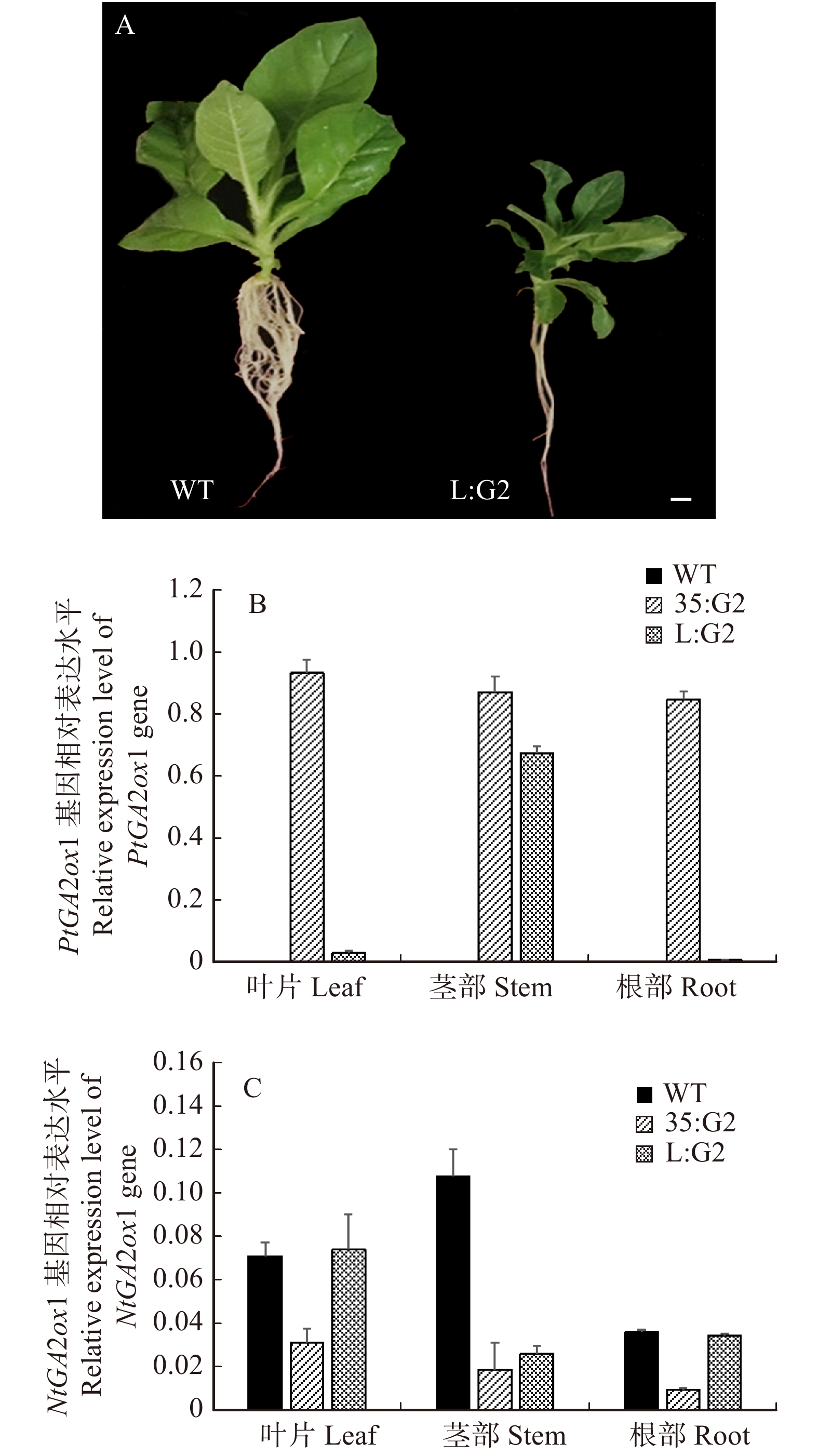
通过农杆菌介导的转基因技术获得了以木质部形成层特异性启动子LMX5介导的烟草转基因株系L:G2,提取植株的DNA进行PCR扩增反应进行初步鉴定,成功获得21个转基因株系,与野生型WT相比,转基因植株(图1A)生长矮小,不定根数目减少,差异明显。
![]() 图 1 不同烟草植株及基因表达水平A是不同烟草植株,标尺长度为1 cm;B是转基因株系各组织中PtGA2ox1基因的表达水平;C是转基因株系不同组织中烟草NtGA2ox1基因本底表达水平,将35:G2植株中的PtGA2ox1基因的表达量设置为1。A is different tobacco plant with a scale length of 1 cm; B is the expression level of PtGA2ox1 gene in each tissue of transgenic lines; C is the background expression level of tobacco NtGA2ox1 gene in different tissues of transgenic lines, which will be in 35:G2 plants, the expression level of the PtGA2ox1 gene was set to 1.Figure 1. Different tobacco plants and gene expression levels
图 1 不同烟草植株及基因表达水平A是不同烟草植株,标尺长度为1 cm;B是转基因株系各组织中PtGA2ox1基因的表达水平;C是转基因株系不同组织中烟草NtGA2ox1基因本底表达水平,将35:G2植株中的PtGA2ox1基因的表达量设置为1。A is different tobacco plant with a scale length of 1 cm; B is the expression level of PtGA2ox1 gene in each tissue of transgenic lines; C is the background expression level of tobacco NtGA2ox1 gene in different tissues of transgenic lines, which will be in 35:G2 plants, the expression level of the PtGA2ox1 gene was set to 1.Figure 1. Different tobacco plants and gene expression levels通过测定转基因烟草L:G2、35:G2外源基因的表达水平(图1B、1C),发现L:G2、35:G2转基因植株的表达模式不同,PtGA2ox1转录水平只在L:G2的茎部出现了明显的上升趋势,而叶片和根部的上升趋势不明显。通过测定转基因烟草的叶片、根部及茎部中NtGA2ox1基因的本底表达水平,发现转基因烟草株系L:G2在叶片和根部受自身调控的NtGA2ox1基因表达水平与野生型WT对照比并无明显变化,而茎部NtGA2ox1基因的表达水平和野生型WT相比却明显降低;同时发现转基因烟草株系35:G2各组织中的NtGA2ox1基因表达水平与WT相比均有明显的下降。
在烟草中,主要活性赤霉素GA1在L:G2的茎部含量明显低于野生型WT植株,而非活性的GA8在茎部的含量与WT相比有显著的提高(图2);而转基因株系35:G20与WT相比,根、茎以及叶片内活性GAs(主要是GA1)含量显著提高,即植株整体GAs含量上调(图2);转基因植株35:G2茎、叶部分的活性GAs含量均有显著的下调,而非活性GAs含量增多,植株整体GAs含量下调,可能是GAs含量下降负反馈调节导致活性GA2-氧化酶含量下降 (图2)。
2.2 活性GAs在烟草茎中的运输特性
对35:G20//35:G2、35:G2//35:G20、及WT//WT 3组嫁接苗进行观察,发现接穗和砧木的表型性状与其各自基因型植株的性状并无较大差异(图3)。表明35:G20植株体内产生的大量活性GAs无法在嫁接植株中自由转运到35:G2中,而35:G2植株中的GA2-氧化酶也无法转化35:G20中的大量活性GAs;但培养在添加有GA4+7培养基中的WT//WT嫁接植株,相同生长时间内接穗和砧木的性状都受GAs影响,与35:G20表型相似,均表现为生长迅速,节间和叶片快速伸长,叶片颜色呈浅绿色细长的状态。
![]() 图 3 不同基因型组成的嫁接苗和不同基因型的烟草植株A.普通MS培养基中的WT//WT嫁接植株; B.添加有GA4+7的WT//WT嫁接植株; C.普通MS培养基中的35:G2//35:G20嫁接植株; D.普通MS培养基中的35:G20//35:G2嫁接植株; E.不同基因型的烟草植株。箭头代表嫁接处;A ~ D标尺长度为2 cm;E标尺长度为1 cm。The E scale is 1 cm in length; A,WT//WT grafted plants in normal MS medium; B,WT//WT grafted plants with GA4+7 were added; C, G2//35: G20 grafted plants in normal MS medium; D, 35:G20//35:G2 grafted plants in normal MS medium; E,different genotypes of tobacco plants. The arrow represents the graft; the A−D scale is 2 cm in length.Figure 3. Grafted seedlings of different genotypes and tobacco plants of different genotypes
图 3 不同基因型组成的嫁接苗和不同基因型的烟草植株A.普通MS培养基中的WT//WT嫁接植株; B.添加有GA4+7的WT//WT嫁接植株; C.普通MS培养基中的35:G2//35:G20嫁接植株; D.普通MS培养基中的35:G20//35:G2嫁接植株; E.不同基因型的烟草植株。箭头代表嫁接处;A ~ D标尺长度为2 cm;E标尺长度为1 cm。The E scale is 1 cm in length; A,WT//WT grafted plants in normal MS medium; B,WT//WT grafted plants with GA4+7 were added; C, G2//35: G20 grafted plants in normal MS medium; D, 35:G20//35:G2 grafted plants in normal MS medium; E,different genotypes of tobacco plants. The arrow represents the graft; the A−D scale is 2 cm in length.Figure 3. Grafted seedlings of different genotypes and tobacco plants of different genotypes2.3 下调烟草形成层GAs对茎部木质部生长发育的影响
通过转基因方法上调GAs含量促进植株(35:G20)生长,而下调GA含量的植株(35:G2、L:G2)生长缓慢[7],且下调植株整体GAs含量(35:G2)与特异下调形成层中的GAs含量对植株(L:G2)木质部发育的影响程度相似,说明植株GAs含量下降会使木质部的生长发育变延缓,植株的伸长生长也会受到抑制。通过茎部切片观察(图4),发现上调植株内源GAs含量(35:G20),木质部成熟提前,细胞排列整齐紧密且层数增加;下调GAs含量(35:G2、L:G2),木质部分化滞后,成熟缓慢,依据染色区域的大小发现同时期茎部相同位置的初生木质部多于WT,且细胞排列疏松不规则(图4)。转基因植株35:G2与L:G2无论是茎顶部亦或是茎基部组织中木质部的形成与发育的滞后程度、木质部细胞的排列方式等都极为相似,且下调木质部形成层的GAs含量可以达到下调植株整体GAs含量对木质部发育的影响,说明GAs影响植株木质部生长发育主要是在其形成层中发挥作用,形成层中的GAs含量对植株木质部的生长发育起关键作用。
2.4 下调烟草形成层GAs对叶片生长的影响
对转基因植株和WT的叶片进行观察,发现L:G2和35:G2的叶片均呈墨绿色且生长紧密,且L:G2的叶片数有一定增加(图5)。此外,L:G2的叶片与其他株系相比出现生长不对称和皱化的现象,推测可能是形成层内GAs含量的变化调控了植株木质部的生长,根据叶片面积发生的明显变化判断当特异性降低形成层GAs的含量时延缓了叶脉的正常发育(图5)。
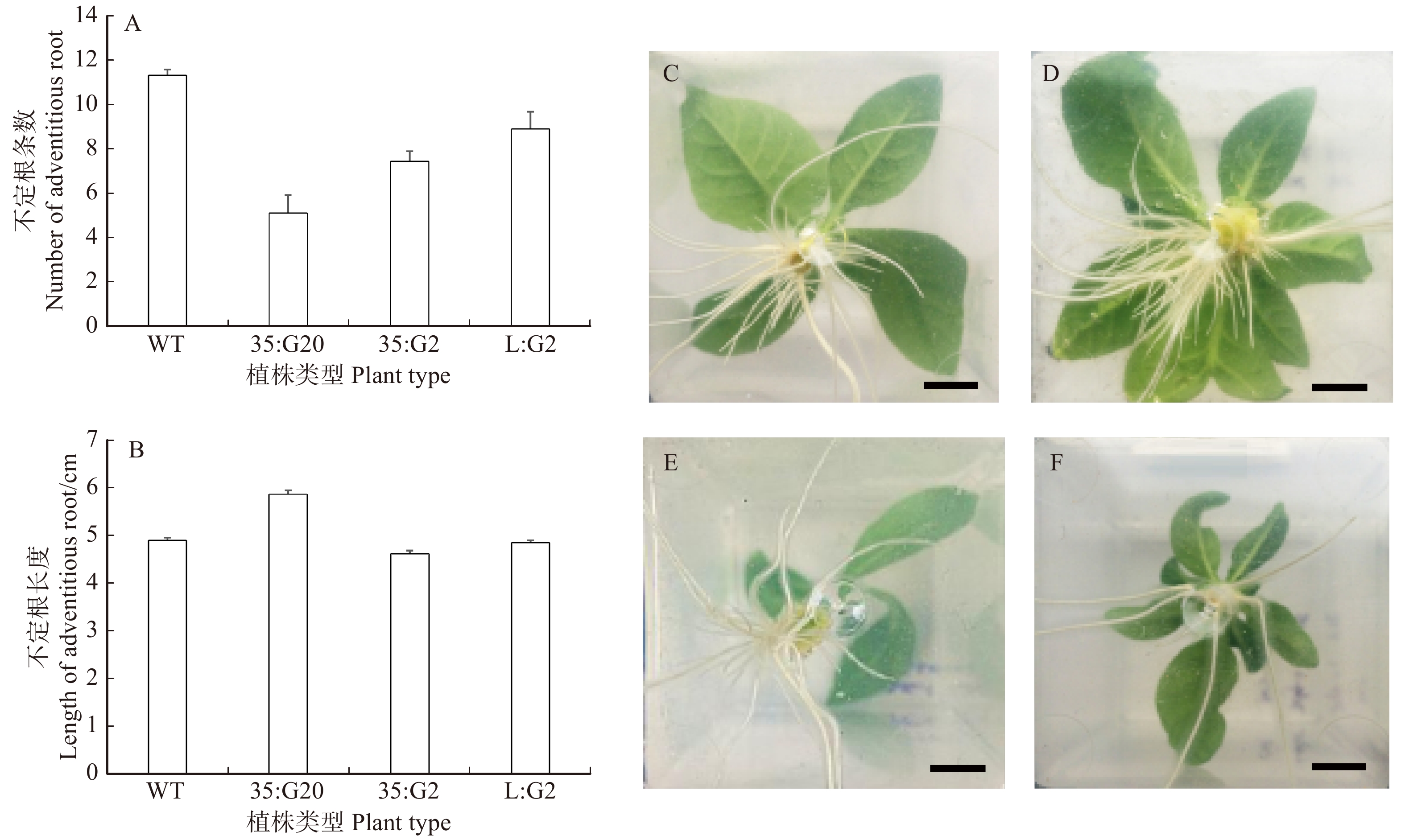
2.5 下调烟草形成层GAs对不定根的影响
与对照组相比,不定根的数量在不同程度上均有所减少(图6A),通过t检验,发现其在0.01水平上差异显著,说明GAs含量的升高或降低对不定根的发育均有显著影响[9]。L:G2株系和35:G2株系不定根的生根性状在不定根数量和长度方面均相似(图6A、6B),表明特异性降低形成层中GAs的含量与降低植株整体GAs的含量对植株不定根的发育影响程度几乎相同,即木质部形成层中的活性GAs的含量是影响不定根发育的主要因素之一。
![]() 图 6 内源调控GAs含量对植株根生长发育的影响A、B为野生型和转基因植物不定根的平均数量和平均长度;C ~ F为不同转基因型植株根部,标尺长度为1 cm。C为WT株系;D为35:G2株系;E为35:G20株系;F为L:G2株系。A, B, the number and length of average adventitious roots of wild-type and transgenic plants. C−F, roots of different transgenic plants, the length of the scale is 1 cm. C, WT strain; D, 35: G2 strain; E, 35: G20 strain; F, L: G2 strain.Figure 6. Effects of endogenous regulation of GAs content on root growth and development of plants
图 6 内源调控GAs含量对植株根生长发育的影响A、B为野生型和转基因植物不定根的平均数量和平均长度;C ~ F为不同转基因型植株根部,标尺长度为1 cm。C为WT株系;D为35:G2株系;E为35:G20株系;F为L:G2株系。A, B, the number and length of average adventitious roots of wild-type and transgenic plants. C−F, roots of different transgenic plants, the length of the scale is 1 cm. C, WT strain; D, 35: G2 strain; E, 35: G20 strain; F, L: G2 strain.Figure 6. Effects of endogenous regulation of GAs content on root growth and development of plants在植株的侧根生长性状方面(图6C ~ 6F),L:G2株系几乎没有侧根产生,而35:G2的侧根与野生型WT相比有明显的增多,进一步说明特异性下调形成层内活性GAs含量对植株生长发育具有一定的调控作用,会抑制侧根的发生。
3. 讨 论
已有研究表明,赤霉素对茎部形成层、根尖的生长[9]及抗低温胁迫都有调控作用,可促进植物的生长[11-12],然而相关研究皆是以施加外源GA3或上调GAs含量为实验依据,以全株组成型调控赤霉素含量进行分析,未考虑活性赤霉素在活体内的运输特性对研究结果的影响。本研究通过对转基因及野生型烟草进行大量的观察与分析,发现特异性下调形成层赤霉素含量对植物的生长会有一定影响,如叶片不能正常伸展,木质部发育延缓,侧根和不定根的发生也会受到抑制。说明特异性调控植物局部的GAs含量对植株整体的生长发育造成了影响,且特异下调形成层区域的GAs可以达到下调植株整体GAs对木质部发育影响的效果,表明GAs在形成层中发挥了关键调控作用。
此外,利用烟草微嫁接技术将两种不同基因型的烟草嫁接后依然能生根并进行营养生长,且接穗与砧木的生长表型分别与其自身表型相同,说明活性内源GAs并不能在二者之间自由运输;而将WT//WT嫁接苗在添加有GA4+7的培养基中培养,发现嫁接苗的接穗与砧木都表现为GAs含量上调植株的表型,说明了外源的GAs通过根部吸收后,又可在体内自由运输。从以上两种结果可以判断,内源GAs和外源GAs在植物体内的运输途径可能不同,外源施加的活性GAS可能并不是通过主动运输而是通过导管组织随矿物质进行运输,而内源激素是通过形成层进行运输,这就是为什么微嫁接后内源活性GAs无法在植物体内自由运输的原因,具体机制有待于进一步的深入解析。本研究系统地证实了特异性调控形成层GAs含量在植株生长发育调控中的重要作用,为茎部赤霉素含量对植物生长发育的特异性调控机制的研究提供了理论基础,特别是赤霉素对木质部分化的特异性调控作用,为其在林木育种中的实际应用提供了依据。
-
图 1 不同烟草植株及基因表达水平
A是不同烟草植株,标尺长度为1 cm;B是转基因株系各组织中PtGA2ox1基因的表达水平;C是转基因株系不同组织中烟草NtGA2ox1基因本底表达水平,将35:G2植株中的PtGA2ox1基因的表达量设置为1。A is different tobacco plant with a scale length of 1 cm; B is the expression level of PtGA2ox1 gene in each tissue of transgenic lines; C is the background expression level of tobacco NtGA2ox1 gene in different tissues of transgenic lines, which will be in 35:G2 plants, the expression level of the PtGA2ox1 gene was set to 1.
Figure 1. Different tobacco plants and gene expression levels
图 3 不同基因型组成的嫁接苗和不同基因型的烟草植株
A.普通MS培养基中的WT//WT嫁接植株; B.添加有GA4+7的WT//WT嫁接植株; C.普通MS培养基中的35:G2//35:G20嫁接植株; D.普通MS培养基中的35:G20//35:G2嫁接植株; E.不同基因型的烟草植株。箭头代表嫁接处;A ~ D标尺长度为2 cm;E标尺长度为1 cm。The E scale is 1 cm in length; A,WT//WT grafted plants in normal MS medium; B,WT//WT grafted plants with GA4+7 were added; C, G2//35: G20 grafted plants in normal MS medium; D, 35:G20//35:G2 grafted plants in normal MS medium; E,different genotypes of tobacco plants. The arrow represents the graft; the A−D scale is 2 cm in length.
Figure 3. Grafted seedlings of different genotypes and tobacco plants of different genotypes
图 6 内源调控GAs含量对植株根生长发育的影响
A、B为野生型和转基因植物不定根的平均数量和平均长度;C ~ F为不同转基因型植株根部,标尺长度为1 cm。C为WT株系;D为35:G2株系;E为35:G20株系;F为L:G2株系。A, B, the number and length of average adventitious roots of wild-type and transgenic plants. C−F, roots of different transgenic plants, the length of the scale is 1 cm. C, WT strain; D, 35: G2 strain; E, 35: G20 strain; F, L: G2 strain.
Figure 6. Effects of endogenous regulation of GAs content on root growth and development of plants
-
[1] Stefano G, Annalisa R, Julie M, et al. The Dof protein DAG1 mediates PIL5 activity on seed germination by negatively regulating GA biosynthetic gene AtGA3ox1[J]. The Plant Journal, 2010, 61(2): 312−323.
[2] Mikihiro O, Atsushi H, Yukika Y, et al. Gibberellin biosynthesis and response during Arabidopsis seed germination[J]. The Plant Cell, 2003, 15(7): 1591−1604. doi: 10.1105/tpc.011650
[3] Bleecker A B, Schuette J L, Kende H. Anatomical analysis of growth and developmental patterns in the internode of deepwater rice[J]. Planta, 1986, 169(4): 490−497. doi: 10.1007/BF00392097
[4] van der Knaap E, Kim J H, Kende H. A novel gibberellin-induced gene from rice and its potential regulatory role in stem growth[J]. Plant Physiology, 2000, 122(3): 695−704. doi: 10.1104/pp.122.3.695
[5] 季兰, 杨仁崔. 水稻茎伸长生长与植物激素[J]. 植物学报, 2002, 19(1):109−115. doi: 10.3969/j.issn.1674-3466.2002.01.016 Ji L, Yang R C. Rice stem elongation and plant hormones[J]. Chinese Bulletin of Botany, 2002, 19(1): 109−115. doi: 10.3969/j.issn.1674-3466.2002.01.016
[6] Stamm P, Kumar P P. Auxin and gibberellin responsive Arabidopsis SMALL AUXIN UP RNA36 regulates hypocotyl elongation in the light[J]. Plant Cell Reports, 2013, 32(6): 759−769. doi: 10.1007/s00299-013-1406-5
[7] 李哲馨, 钮世辉, 高琼, 等. 赤霉素调控木质部发育的细胞学研究[J]. 北京林业大学学报, 2014, 36(2):68−73. Li Z X, Niu S H, Gao Q, et al. Cytological study of gibberellin regulated xylem development[J]. Journal of Beijing Forestry University, 2014, 36(2): 68−73.
[8] Ragni L, Nieminen K, Pacheco-Villalobos D, et al. Mobile gibberellin directly stimulates Arabidopsis hypocotyl xylem expansion[J]. Plant Cell, 2011, 23(4): 1322−1336. doi: 10.1105/tpc.111.084020
[9] Niu S, Li Z, Yuan H, et al. Proper gibberellin localization in vascular tissue is required to regulate adventitious root development in tobacco[J]. Journal of Experimental Botany, 2013, 64(11): 3411−3424. doi: 10.1093/jxb/ert186
[10] 钮世辉, 李伟, 陈晓阳. 赤霉素对根尖径向生长的调节作用研究[J]. 北京林业大学学报, 2013, 35(3):71−76. Niu S H, Li W, Chen X Y. Negative regulation of gibberellin on root tip diameter[J]. Journal of Beijing Forestry University, 2013, 35(3): 71−76.
[11] Niu S, Gao Q, Li Z, et al. The role of gibberellin in the CBF1-mediated stress-response pathway[J]. Plant Molecular Biology Reporter, 2014, 32(4): 852−863. doi: 10.1007/s11105-013-0693-x
[12] 高琼, 钮世辉, 李伟, 等. 低温胁迫对赤霉素代谢的调控研究[J]. 北京林业大学学报, 2014, 36(6):135−141. Gao Q, Niu S H, Li W, et al. Regulation of low temperature stress on gibberellin metabolism[J]. Journal of Beijing Forestry University, 2014, 36(6): 135−141.
[13] 魏佳玉, 张素芳, 刘紫怡, 等. 长白落叶松形成层差异表达的miRNA[J]. 东北林业大学学报, 2018, 46(6):14−18. doi: 10.3969/j.issn.1000-5382.2018.06.003 Wei J Y, Zhang S F, Liu Z Y, et al. Differentially expressed miRNA of Larix olgensis cambium[J]. Journal of Northeast Forestry University, 2018, 46(6): 14−18. doi: 10.3969/j.issn.1000-5382.2018.06.003
[14] 张凤娟. 外源GA对垂柳茎部次生木质部和次生韧皮部发生的影响(简报)[J]. 河北农业技术师范学院学报, 1997, 11(4):74−76. Zhang F J. Effects of external GA on second-xylem and second-phloem of weeping willow stems (bulletin)[J]. Journal of Hebei Agrotechnical Teachers College, 1997, 11(4): 74−76.
[15] 刘畅. 形成层赤霉素含量对烟草发育的影响[D].北京: 北京林业大学, 2016. Liu C. Roles of the cambial GA concentration on tobacco development[D]. Beijing: Beijing Forestry University, 2016.
[16] Regnault T, Davière J M, Wild M, et al. The gibberellin precursor GA12 acts as a long-distance growth signal in Arabidopsis[J/OL]. Nature Plants. 2015, 1: 15073 [2019−01−21]. https://doi.org/10.1038/nplants.2015.73.
[17] Turnbull C G, Booker J P, Leyser H M. Micrografting techniques for testing long-distance signalling in Arabidopsis[J]. Plant Journal, 2002, 32(2): 255−262. doi: 10.1046/j.1365-313X.2002.01419.x
[18] Love J, Bjorklund S, Vahala J, et al. Ethylene is an endogenous stimulator of cell division in the cambial meristem of Populus[J]. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(14): 5984−5989. doi: 10.1073/pnas.0811660106
[19] Gou J, Strauss S H, Tsai C J, et al. Gibberellins regulate lateral root formation in Populus through interactions with auxin and other hormones[J]. The Plant Cell, 2010, 22(3): 623−639. doi: 10.1105/tpc.109.073239
-
期刊类型引用(2)
1. 宋晴,付鸿莉,王铁梅,宿逸然,梁留喜,通拉嘎,胥健,董昊野,邰塔拉. 兴安盟草原灌丛植被潜在适生区分布模拟分析. 草地学报. 2024(02): 579-587 .  百度学术
百度学术
2. 楼科尔,曲文杰,王磊,王兴,郜永贵,张波,尤万学,杨新国. 腾格里沙漠地区4种优势一年生草本植物根构型特征. 应用生态学报. 2024(11): 3015-3022 .  百度学术
百度学术
其他类型引用(1)



 下载:
下载: