Cloning the promoter of BpSPL8 from Betula platyphylla and overexpression of BpSPL8 gene affecting drought tolerance in Arabidopsis thaliana
-
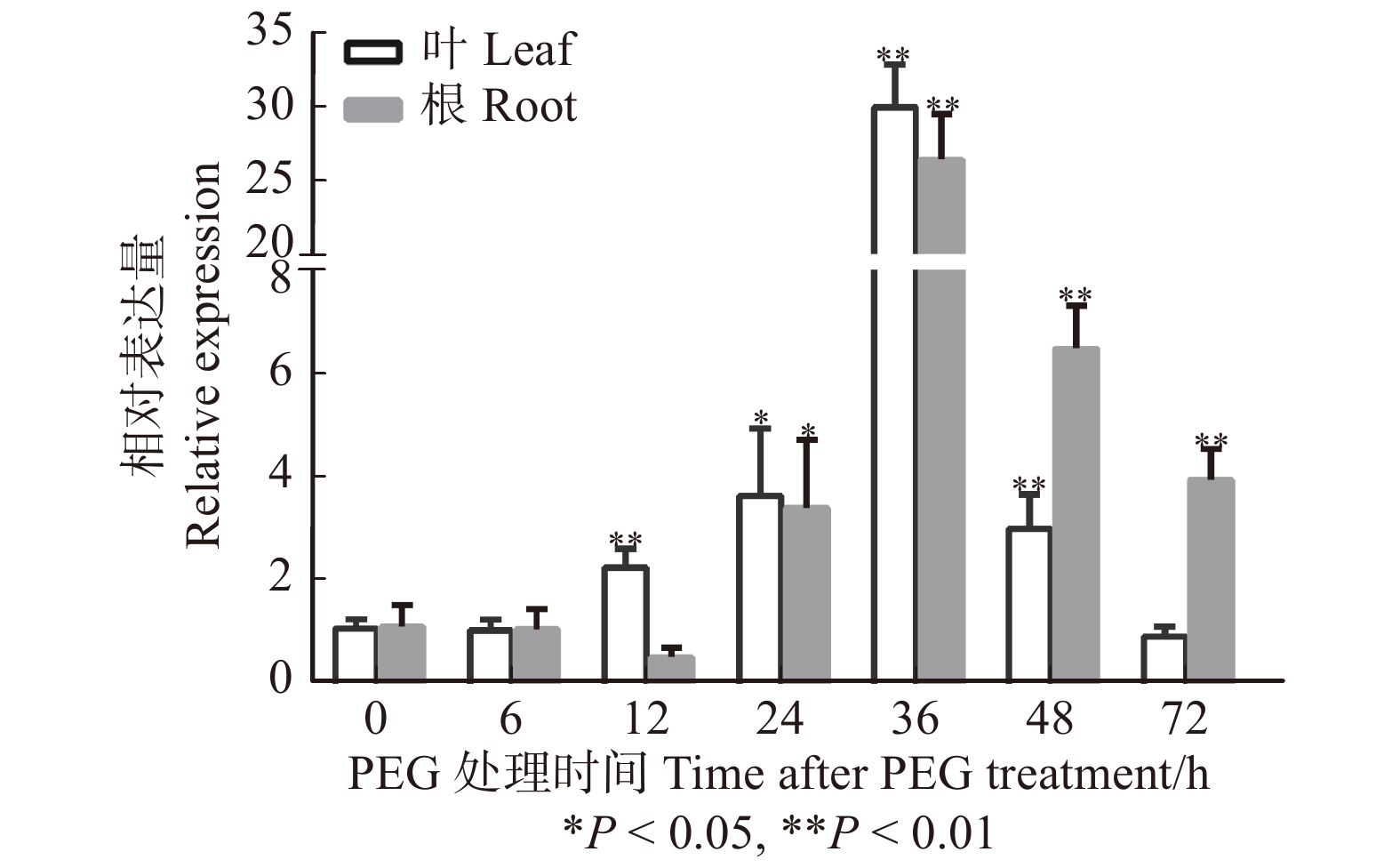
摘要:目的目前对植物SPL8的基因功能研究主要集中在开花和育性方面,而其在干旱胁迫响应中的作用却鲜有报道。本文克隆、分析了白桦BpSPL8启动子,并研究了BpSPL8基因在拟南芥中响应干旱胁迫的功能。方法通过PCR克隆技术得到了白桦BpSPL8启动子;利用PLACE和PlantCARE软件对BpSPL8启动子顺式作用元件进行了预测。构建了BpSPL8启动子驱动GUS(β-葡萄糖苷酸酶编码基因)的植物表达载体,并采用浸花法将其转化至拟南芥中;继而利用GUS染色分析了BpSPL8启动子的组织表达模式;同时对BpSPL8在PEG处理下的表达水平进行了qRT-PCR分析。最后,以过表达BpSPL8拟南芥为材料来探究BpSPL8在干旱胁迫下的生物学功能。结果启动子元件分析显示,BpSPL8启动子中含有组织特异表达、光响应、激素响应及多个胁迫响应元件。GUS染色结果表明,BpSPL8启动子可在拟南芥的下胚轴、叶片、叶柄、根和花序中启动GUS基因表达。BpSPL8基因在PEG处理下的野生型白桦的根和叶片中均呈现先上调后下调的表达趋势。干旱胁迫下,过表达BpSPL8拟南芥的存活率和脯氨酸含量均显著低于野生型,丙二醛含量显著高于野生型;两个已知的抗逆基因DR29B和P5CS1在干旱处理后的野生型和转基因拟南芥中均上调表达;但在转基因拟南芥中呈现出延迟上调的表达模式。结论异源过表达白桦BpSPL8能够降低拟南芥的耐旱性,并在干旱胁迫下影响抗性基因DR29B和P5CS1的表达模式。Abstract:ObjectiveCurrently, researches on the gene function of plant SPL8 are mainly focused on flowering and fertility, but there are fewer reports about its SPL8 function in drought stress response. In this paper, BpSPL8 promoter was cloned and analyzed from Betula platyphylla, and the function of BpSPL8 gene in response to drought stress was studied in Arabidopsis thaliana.MethodPromoter sequence of BpSPL8 gene was isolated from Betula platyphylla by PCR technology, and the cis-element prediction of BpSPL8 promoter was performed using PLACE and PlantCARE software. The plant expression vectors with GUS (β-glucuronidase coding gene) expression driven by the promoters of BpSPL8 were constructed and transformed into Arabidopsis thaliana by the floral dip method. Through the detection of GUS activity, the tissue expression pattern of the BpSPL8 promoter in Arabidopsis thaliana was analyzed. QRT-PCR analysis was performed on the expression level of BpSPL8 under PEG treatment. Finally, the overexpression of BpSPL8 Arabidopsis thaliana was used to explore the function of BpSPL8 in the drought process.ResultPromoter element analysis revealed that BpSPL8 promoter contained elements for tissue-specific expression, light-responsive, hormone-responsive and stress-responsive. GUS histochemical staining results showed that GUS activity was observed in hypocotyls, leaves, petioles, roots and inflorescences of transgenic Arabidopsis thaliana carrying the BpSPL8 promoter. The expression patterns of BpSPL8 gene in roots and leaves of birch were up-regulated and then down-regulated under PEG treatment. Drought stress tolerance pointed out that the transgenic plants showed significantly lower survival rate and proline content than wild type, while malondialdehyde content was higher than wild type. Two known stress-resistant genes, DR29B and P5CS1, were up-regulated in wild-type and transgenic Arabidopsis thaliana under drought stress. However, compared with wild-type, they showed delayed up-regulation in transgenic Arabidopsis thaliana.ConclusionEctopic overexpression of BpSPL8 can reduce the drought tolerance of Arabidopsis thaliana and affect the expression patterns of resistance genes DR29B and P5CS1 under drought stress.
-
Keywords:
- promoter /
- BpSPL8 /
- Betula platyphylla /
- transgenic /
- drought
-
转录因子通过调节动物和植物基因的时空表达水平,来调控生物体的细胞增殖、生长发育及免疫反应过程[1]。TCP基因家族编码植物特有的转录调控因子,该家族成员在植物生长[2]、花瓣的不对称性[3-4]、细胞分裂[5]、叶形态发生[6-7]、衰老[8-9]、胚胎生长[10]、昼夜节律[2]中起着关键作用。基于蛋白质的DNA结构域不同,该家族成员分为Class I(PCF类)和Class II(CIN和CYC/TB1)两类。目前,Class I类基因功能在草本植物中研究的较为深入。在拟南芥(Arabidopsis thaliana)中,AtTCP11通过上调VND7基因的表达,引起维管发育缺陷[11];AtTCP14通过细胞增殖控制叶的形态发生;AtTCP20通过抑制茉莉酸(JA)在植物体内的合成,降低其抑制细胞增殖的能力,从而使细胞大小发生改变[12],同时转AtTCP20::EAR拟南芥表现出发芽延迟、茎尖和根尖分生组织生长速度放缓、子叶变黄,下胚轴区域膨胀等特征[13]。
TCP基因参与多种激素信号传递过程,调控植物形态发育。在拟南芥中,AtTCP5、AtTCP13和AtTCP17通过PIFs依赖途径和不依赖PIFs途径两种方式来促进生长素的合成,进而介导拟南芥下胚轴在遮荫条件下的伸长[14];AtTCP3和AtTCP15通过调控生长素响应相关基因SHY2/IAA3和SAUR的表达参与生长素信号的转导[15-16];AtTCP9通过改变JA的代谢从而改变根长[17];在陆地棉(Gossypium hirsutum)中,GhTCP1基因通过促进JA的合成而使棉花纤维伸长并促进根毛发育[18]。AtTCP14和AtTCP15参与细胞分裂素(CK)信号途径的应答,影响细胞分裂[19];I类TCP转录因子家族参与赤霉素(GA)信号转导,研究发现GA通过刺激DELLA蛋白的降解来控制茎和节间伸长,而DELLA蛋白直接调节植物特异性I类TCP转录因子家族的活性,从而控制细胞增殖,同时拟南芥tcp8、tcp14、tcp15、tcp22这4个基因的突变体表现出严重的侏儒症和对GA作用的反应敏感性降低的性状[20]。目前对PCF亚类基因的研究主要集中在草本植物上,而在木本植物中研究较少。
白桦(Betula platyphylla),桦木科(Betulaceae)落叶类乔木,白桦树干修直、洁白,且耐严寒,生长较快,是我国东北地区珍贵阔叶树种之一。本研究以白桦为试材,克隆了BpTCP2基因编码区的全长序列,对其进行生物信息学分析,同时采用qRT-PCR分析该基因在白桦不同组织部位的表达特征以及对植物激素处理和非生物胁迫的应答机制,为揭示白桦的生长发育、抗逆机制,以及培育优良的白桦新品系提供参考。
1. 材料与方法
1.1 材 料
1.1.1 植物材料
材料于2018年夏季取自东北林业大学林木遗传育种白桦强化育种基地栽植的2年生白桦组培苗木,依次选取5株的顶芽、腋芽、第1到第7茎节(幼嫩到成熟)、成熟茎节的木质部和韧皮部、第1到第13片叶(包括叶片从幼嫩到衰老过程)。以上植物材料经液氮速冻后,放入− 80 ℃超低温冰箱保存,用于RNA的提取和研究BpTCP2在白桦不同组织部位的表达。
从白桦强化育种基地采集的白桦全同胞家系种子通过水培萌发,待长出两片子叶时,分别取10株长势均一的植株,放置在涂有相应外源激素和非生物胁迫试剂的WPM培养基中。激素处理的种类及浓度分别为:50 mg/L IAA,100 μmol/L ABA,0.2 mg/L油菜素类固醇(BR),1 μmol/L JA,350 μmol/L 水杨酸(SA)。非生物胁迫处理的浓度分别为:0.4 mol/L NaCl,0.3 mol/L NaHCO3,150 μmol/L CdCl2,20% PEG。分别在0、2、4、6、12和24 h取材并放置于1.5 mL离心管中,液氮速冻后,放入− 80 ℃超低温冰箱内保存,用于RNA提取。
1.1.2 试 剂
RNA提取试剂盒为通用植物总RNA提取试剂盒(离心柱型),购置于北京百泰克生物技术有限公司,反转录试剂盒为ReverTra Ace® qPCR RT Master Mix with gDNA Remover试剂盒,购置于东洋纺(上海)生物科技有限公司,TransStart Top Green qPCR SuperMix(Perfect Real Time染料法实时荧光定量)购置全式金生物(北京)有限公司,Topo试剂盒为pENTRTM/D-TOPO® Cloning Kit购置于invitrogen公司,大肠杆菌Trans1-T1感受态细胞购自世国生物科技(哈尔滨)有限公司。引物由擎科(哈尔滨)公司合成。
1.2 方 法
1.2.1 白桦BpTCP2基因的克隆
根据白桦基因组和转录组序列比对获得BpTCP2基因的序列设计引物,上、下游引物序列分别为5′-CACCGACATGGCAGAGAGCAAGC-3′、5′-ATTCTTCTACTGCCTTGACC-3′。以白桦不同组织部位的cDNA为模板,PCR扩增BpTCP2基因目标片段,反应体系如下:10 × KOD Buffer 1.7 μL,2 mmol/L dNTPs 1.7 μL,MgSO4 0.8 μL,cDNA模板0.4 μL,上、下游引物各为10 mmol/L 0.6 μL,KOD Plus 0.4 μL,ddH2O补足20 μL。PCR扩增程序为94 ℃预变性2 min;94 ℃变性45 s,58 ℃退火45 s,68 ℃延伸2 min,35个循环;68 ℃延伸10 min。PCR产物经1.0% 琼脂糖凝胶电泳检测,并进行胶回收纯化,将纯化产物进行Topo反应,连接体系为:胶回收产物1 μL,Topo Vector 0.5 μL,Salt Solution 0.5 μL,用ddH2O补足反应体系至3 μL。25 ℃连接30 min,连接反应完成后,通过热击法将连接产物转化到大肠杆菌Trans1-T1感受态细胞中。对获得的单克隆进行PCR检测,条带位置正确后送公司测序,保存经测序对比正确的菌液。
1.2.2 生物信息学分析
采用NCBI ORF Finder查找序列的ORF;用在线软件BioEdit预测该基因氨基酸序列;用Blastx进行同源序列比对,搜索不同物种中的同源基因及蛋白,并先将氨基酸序列利用Clustal X进行多序列比对的分析,借助MEGA 5.1软件的Neighbor-Joining算法,1 000次重复,以默认参数构建系统发育进化树[21]。
1.2.3 Real-time PCR分析BpTCP2基因的表达特性
使用RNA提取试剂盒提取白桦不同组织部位(顶芽、腋芽、木质部、韧皮部、1 ~ 7茎节、1 ~ 13叶片)、5种外源植物激素(IAA、ABA、BR、JA、SA)及4种非生物胁迫(NaCl、NaHCO3、CdCl2、PEG)处理后的整株材料的总RNA,并进行反转录。根据克隆获得的BpTCP2基因的全长cDNA序列设计定量引物,同时选用α-Tubulin作为内参基因[21](引物见表1)。获得的cDNA稀释10倍作为qPCR模板。实时定量PCR反应体系为:6 μL Top qMix,0.24 μL Passive Reference Dye,2 μL cDNA,10 μmol/L的上、下游引物各0.24 μL,用ddH2O补足反应体系至12 μL。扩增反应在ABI PRISM® 7500荧光定量PCR仪上完成,反应程序为94 ℃预变性30 s,94 ℃变性5 s,56 ℃退火15 s,72 ℃延伸34 s(45个循环),绘制溶解曲线的温度为95 ℃持续15 s,60 ℃持续1 min,95 ℃持续30 s。所有样品均进行3次重复,采用– ΔΔCt方法进行基因的相对定量分析。
表 1 实时荧光定量PCR引物序列Table 1. Real-time PCR primer sequences引物名称 Primer name 上游引物序列(5′→3′) Forward primer (5′→3′) 下游引物序列(5′→3′) Reverse primer (5′→3′) BpTCP2 5′-GCTTGCATACAAAGATGGAAGG-3′ 5′-GGAAAAGCTCAATGGACCCAG-3′ α-Tubulin 5′-GCACTGGCCTCCAAGGAT-3′ 5′-TGGGTCGCTCAATGTCAAGG-3′ 2. 结果与分析
2.1 BpTCP2基因的克隆
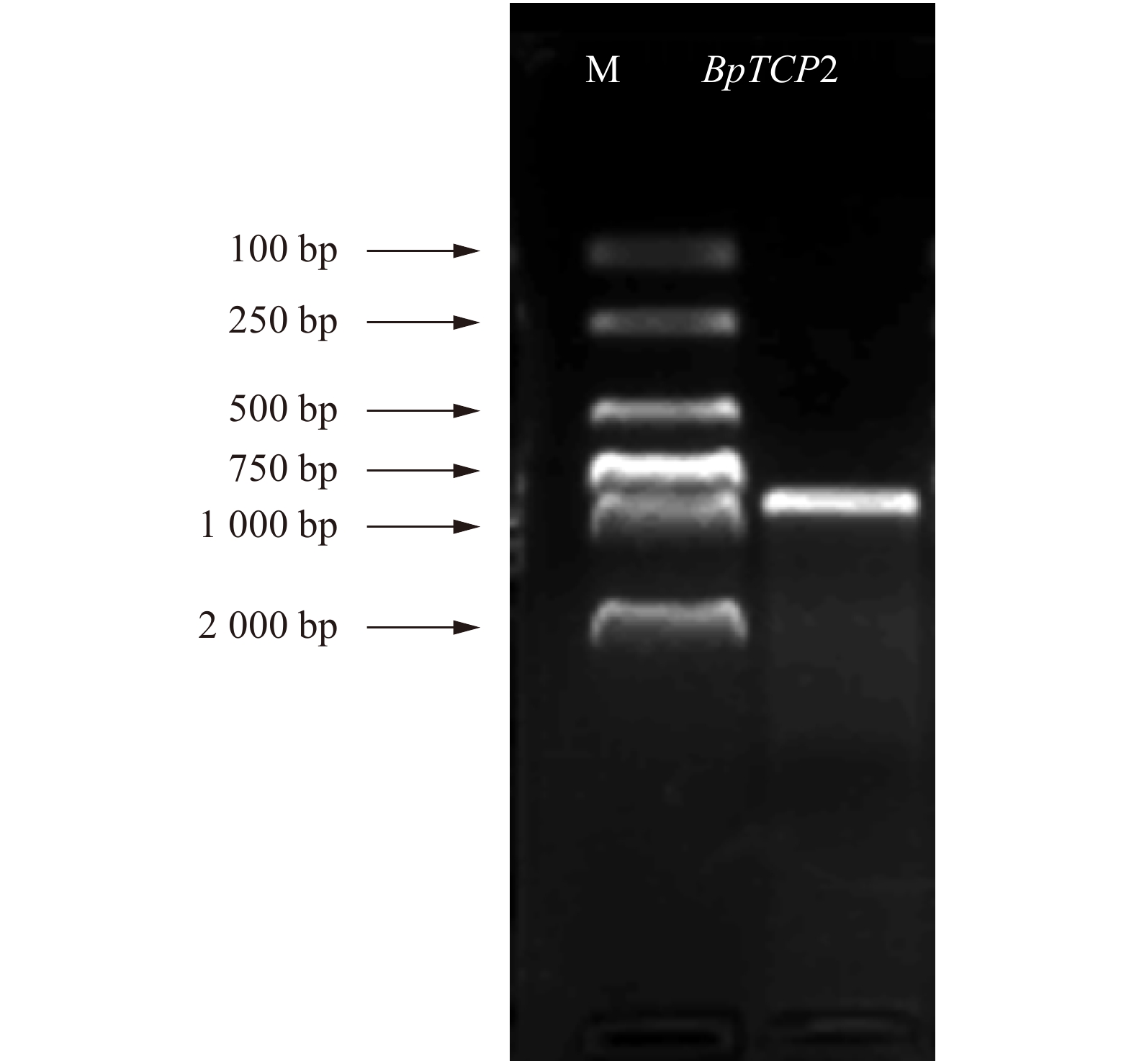
以白桦不同组织部位的cDNA为模板,根据白桦基因组序列和转录组数据比对获得BpTCP2基因序列设计特异性引物,PCR扩增其全长cDNA序列,扩增产物于1.0%琼脂糖凝胶电泳检测(图1),纯化后的胶回收产物通过Topo反应进行菌液PCR检测,结果显示在813 bp处获得特异性条带,与预期目标条带大小一致(图2)。
2.2 白桦BpTCP2基因的生物信息学分析
2.2.1 白桦BpTCP2基因编码区序列分析
利用BLASTx及NCBI ORF Finder预测BpTCP2基因的ORF全长,利用生物学软件BioEdit预测BpTCP2基因的ORF全长为804 bp,编码267个氨基酸(图3)。
2.2.2 白桦BpTCP2基因结构域分析
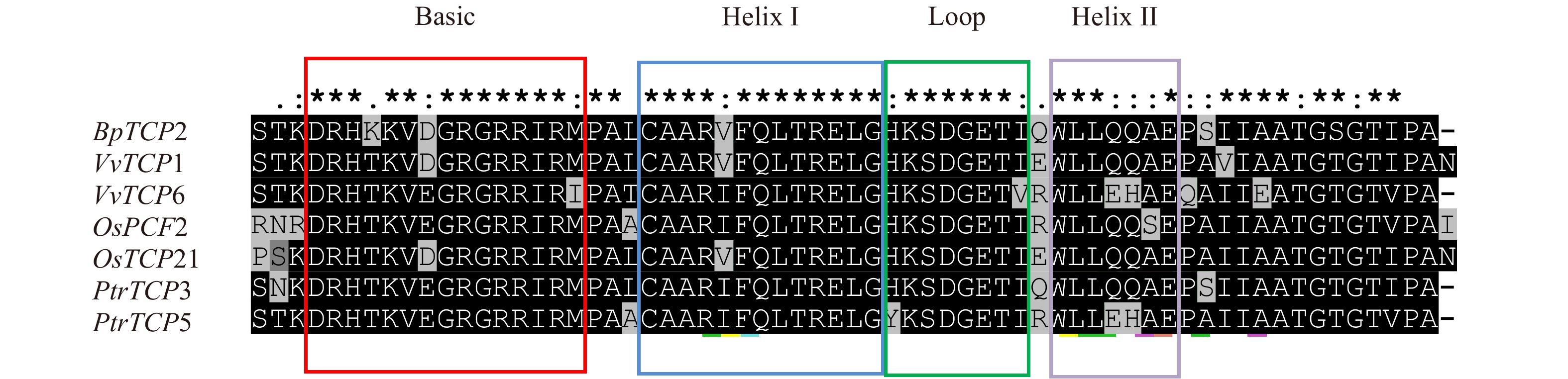
分别选取拟南芥、葡萄(Vitis vinifera)、水稻(Oryza sativa)、毛果杨(Populus trichocarpa)4个物种PCF亚类基因各2条与BpTCP2基因进行氨基酸的多序列比对分析,结果如图4所示,BpTCP2基因与其他物种PCF亚类基因的氨基酸序列均含有TCP保守结构域bHLH[22]。bHLH基序由44个氨基酸组成,包括一个能与DNA结合的碱性区域和α螺旋1-环-α螺旋2(Helix1- Loop-Helix2)组成[23-24],其中Basic结构域含有16个氨基酸,helixI、loop和helixII结构域分别含有11、8和9个氨基酸。从比对结果可以看出无论是Basic结构域还是其他3个结构域均高度保守。
2.2.3 白桦BpTCP2基因的进化树分析
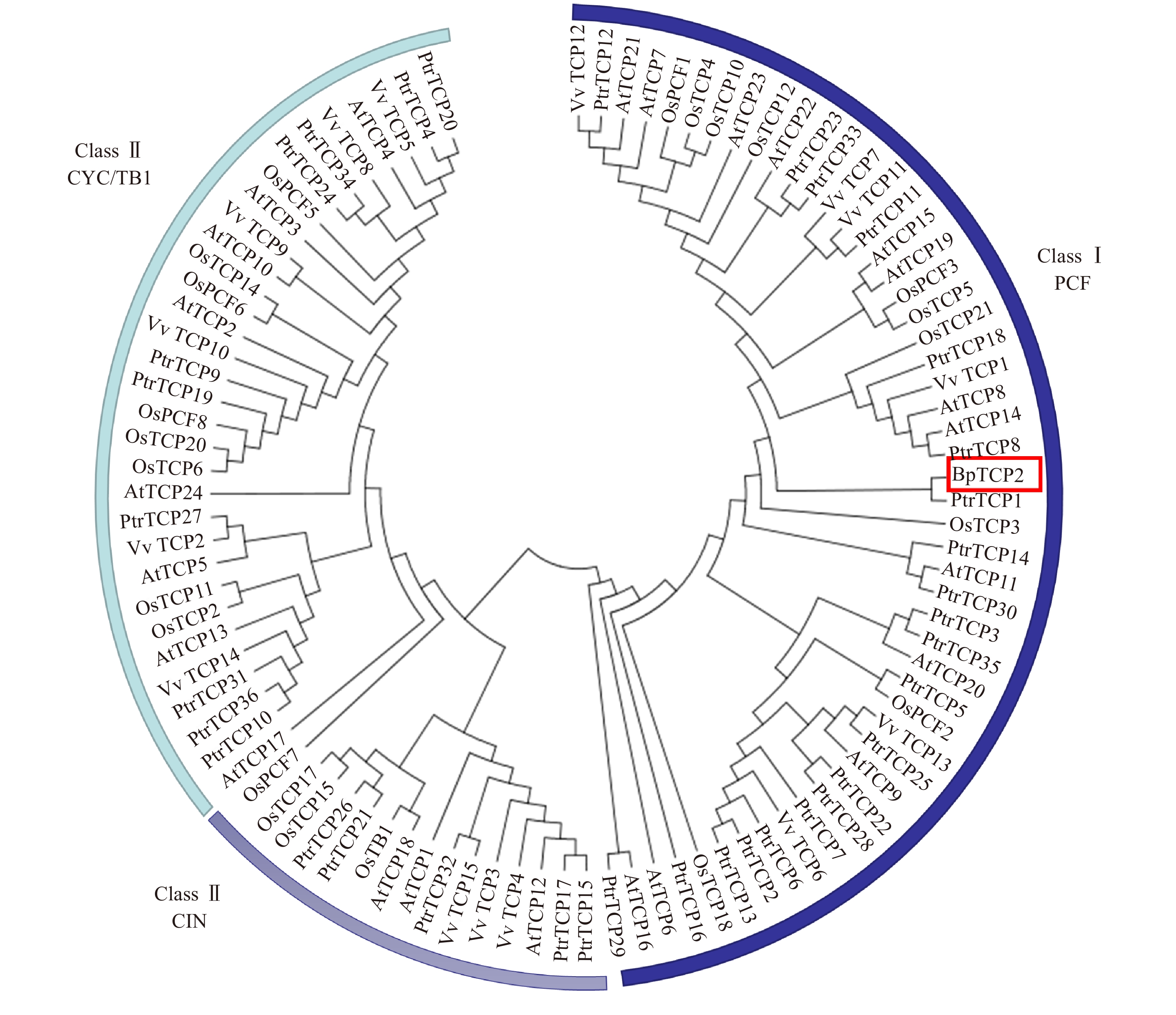
利用MEGA 5.1对白桦BpTCP2与拟南芥、水稻、葡萄及毛果杨TCP的蛋白序列进行聚类分析,结果如图5所示,97个TCP成员被清楚的分为Class I(PCF)和Class II(CIN和CYC/TB1)两大类,其中BpTCP2属于PCF亚类,并与毛果杨的TCP1基因相似性较高。
2.3 BpTCP2基因的组织表达特性分析
2.3.1 不同组织部位
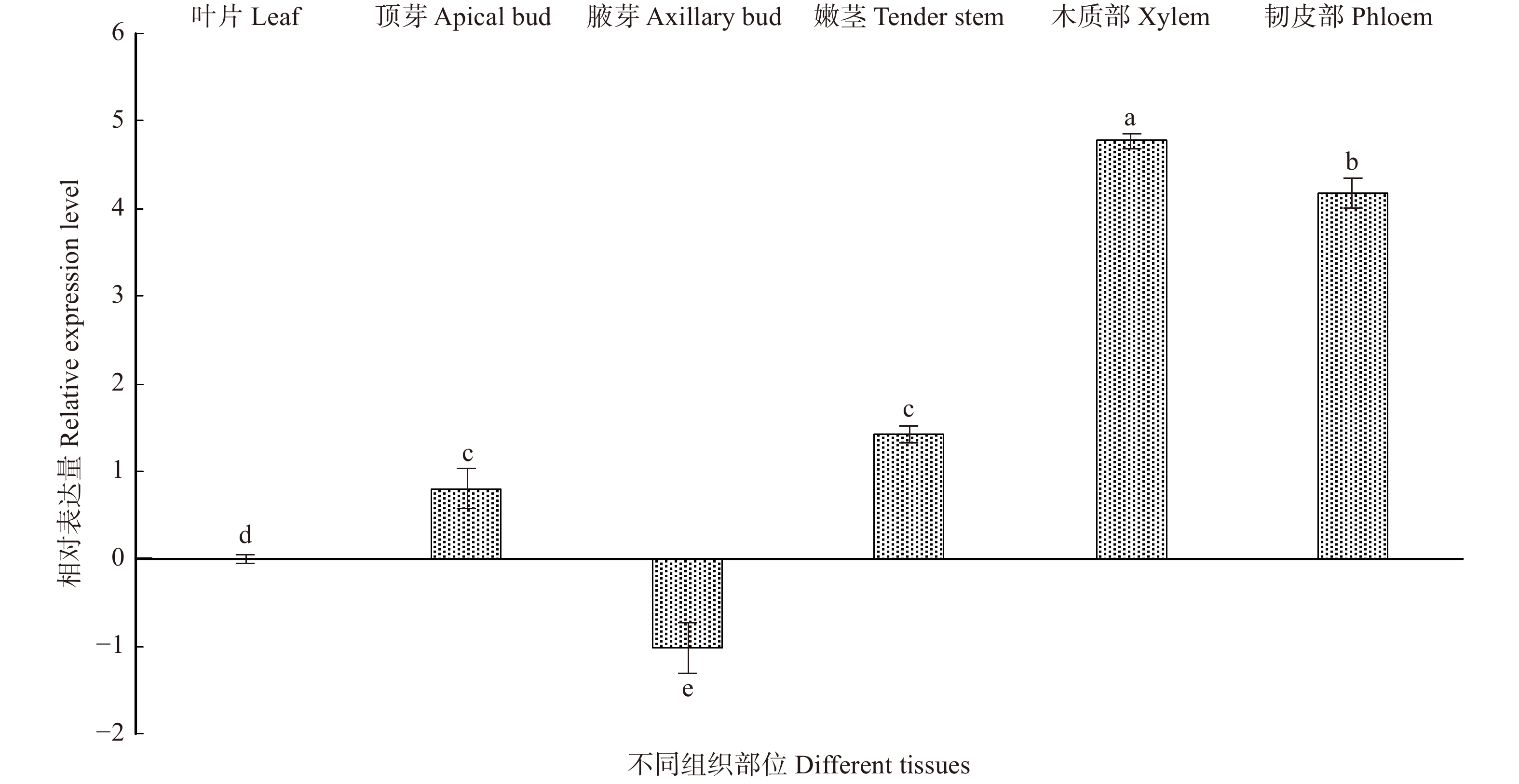
以叶为对照,分析了BpTCP2基因在顶芽、腋芽、嫩茎、木质部及韧皮部的表达情况(图6),结果表明:该基因在腋芽中表达量最低,而在其他4个组织部位中均上调表达,尤其在木质部和韧皮部中,表达量分别上调32倍和16倍。
2.3.2 不同叶发育阶段
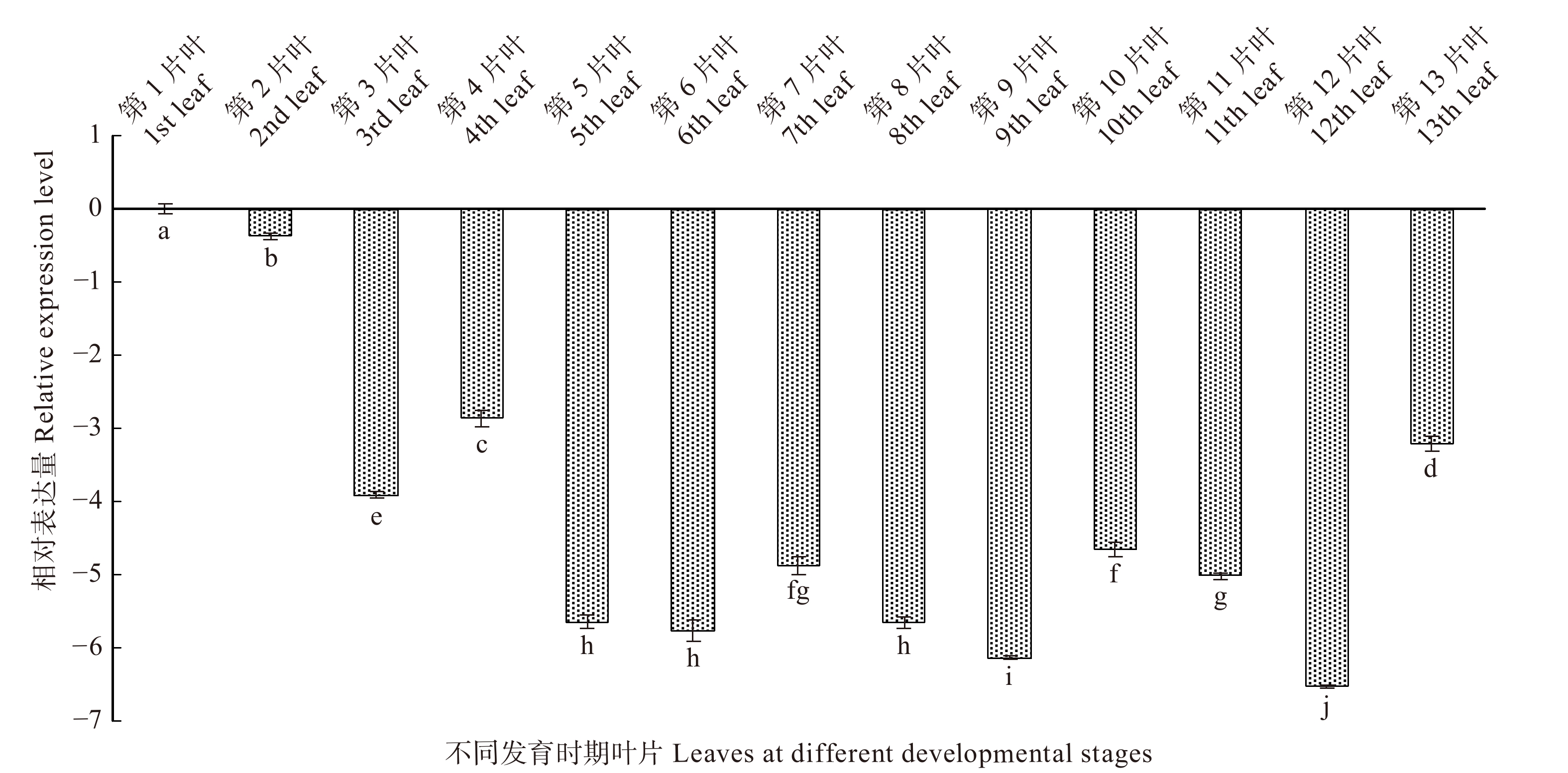
根据白桦叶片的发育情况,分别选取从幼嫩到衰老整个发育阶段的叶片(图7),并以第1片叶为对照,分析了BpTCP2基因在叶片不同发育时期的表达情况(图8),结果表明:该基因随着叶龄的增加整体呈现下调的趋势;在前期发育过程中(第1 ~ 4片叶)BpTCP2表达量呈现先下调后上调的趋势;在中期发育过程(第5 ~ 9片叶),该基因表达量变化并不明显(< 2倍),但低于对照;在后期发育过程(第10 ~ 13片叶)中,该基因持续下调,在第12片叶时下调达到峰值,为对照的128倍,在第13片叶时表达量略有回升。
2.3.3 茎节发育阶段
根据白桦茎节的发育情况,选取了白桦从幼嫩到成熟的茎节如(图9),分析BpTCP2基因在茎节发育阶段的表达情况(图10),以第1茎节为对照,该基因在茎发育初期,表达量呈现持续上调的趋势,尤其是在第4茎节中表达量达到最高峰,为对照的4倍。但从第5茎节到成熟茎节其在韧皮部中的表达量变化不明显(< 2倍)。
2.4 激素应答分析
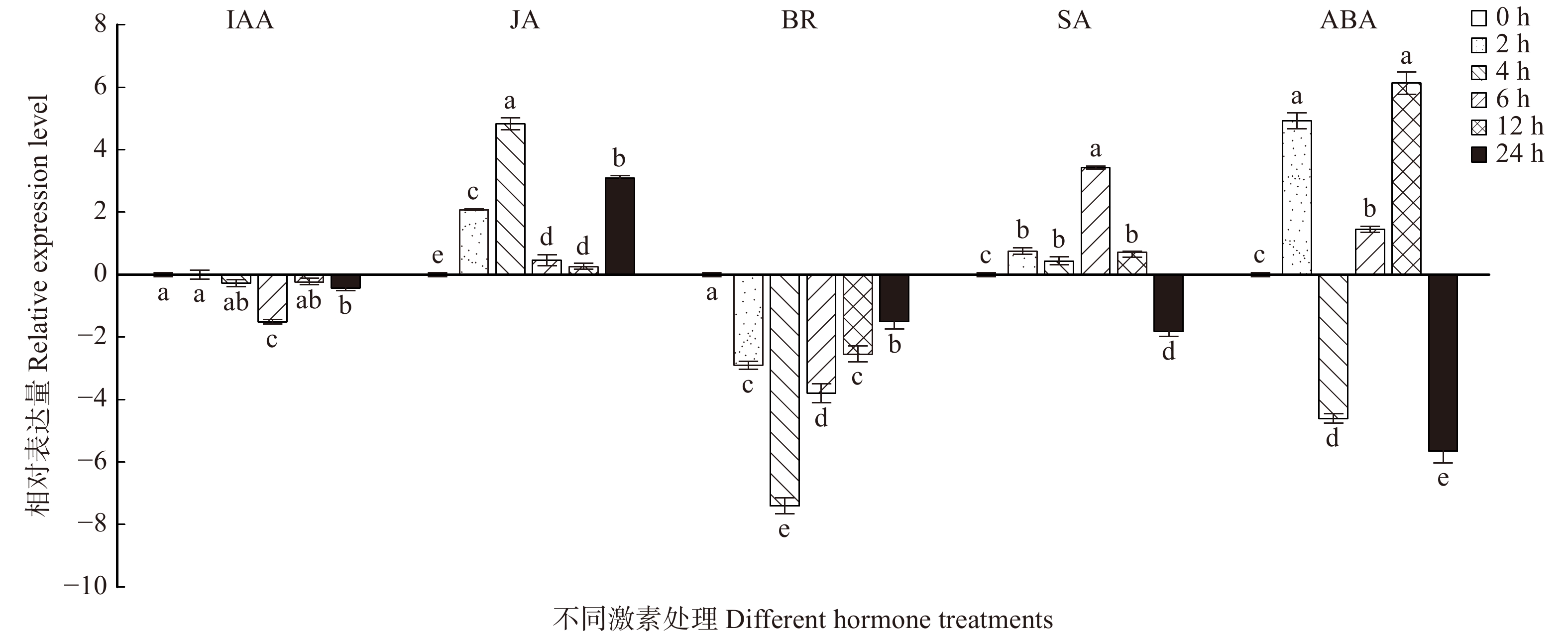
在BR、JA、SA、IAA、ABA这5种激素处理下,BpTCP2的表达情况呈现不同的表达趋势,如图11所示。在BR处理下,BpTCP2基因呈现下调表达的趋势,尤其是在4 h时表达量达到最低值,下调为对照的256倍,随着处理时间的延长,其略有上升但还是低于处理前(0 h);在JA处理过程中,除6、12 h与对照相比变化不明显外,其他时间点均呈现上调表达的趋势,尤其在4 h时,其表达量明显比对照提高了32倍;SA处理后,BpTCP2的表达量在12 h前均上调,且在6 h时达到峰值,为未处理的8倍,而在24 h时骤然下调为4倍;在IAA的处理过程中,BpTCP2基因呈现下调表达的趋势,且除了6 h外,在其他时间点BpTCP2基因的表达量没有发生明显的变化(< 2倍);在ABA处理过程中,BpTCP2的表达量呈现波动,在12 h时表达量达到最高峰,为对照的32倍,而在24 h时表达量最低,为对照的64倍。
2.5 非生物胁迫应答分析
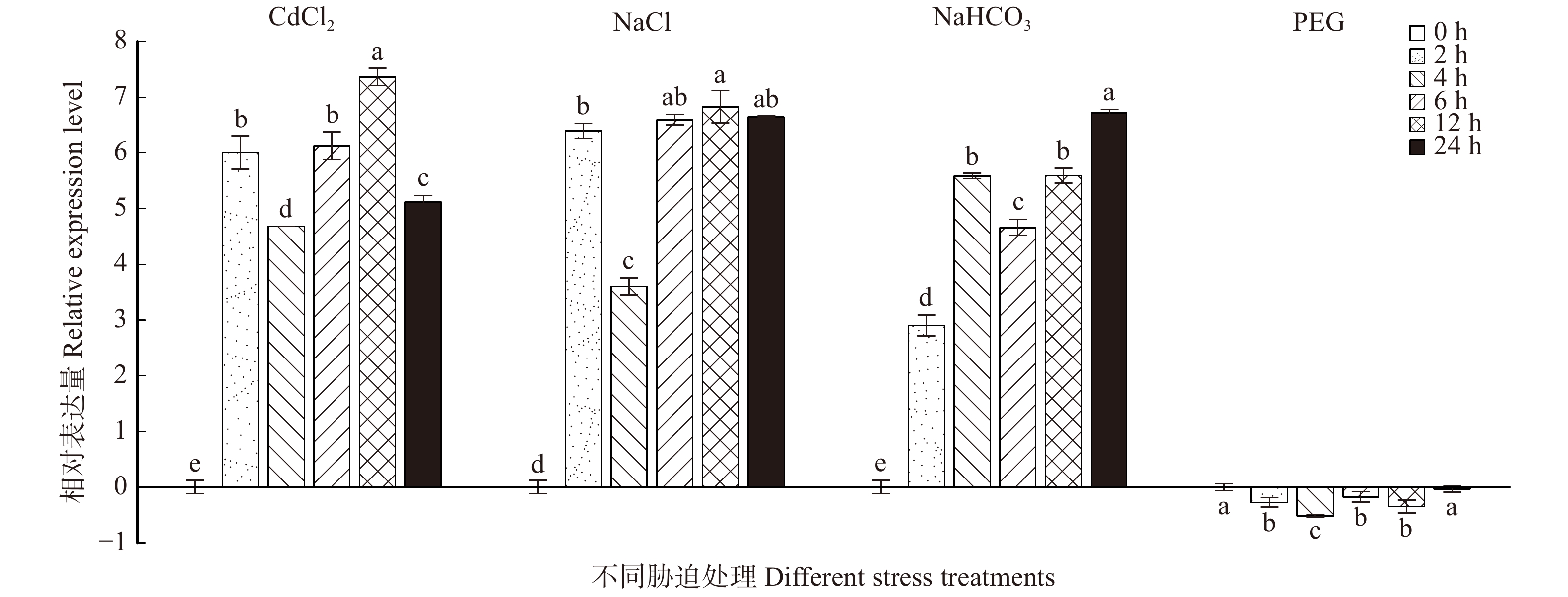
通过CdCl2、NaCl、NaHCO3、PEG 4种胁迫处理,分析BpTCP2的表达情况,结果如图12所示。其中,在CdCl2、NaCl和NaHCO3处理下,BpTCP2基因的表达模式相似且均为高表达,并且CdCl2和NaCl在处理12 h时上调表达量达到峰值,分别为对照的128倍和64倍,NaHCO3处理后表达量最高峰出现在24 h,为对照的64倍;在PEG胁迫处理过程中,BpTCP2与对照相比变化并不明显(< 2倍)。
3. 讨 论
通过对白桦的BpTCP2氨基酸序列以及葡萄、拟南芥、水稻、毛果杨TCP氨基酸序列进行系统聚类分析,根据其序列差异将其分为Class I(PCF)和Class II(CIN和CYC/TB1)两大类,其中BpTCP2属于Class I类PCF亚类。Class I类和Class II类相比,Class I类在Basic结构域上有4个氨基酸的缺失[25-26]。
分析BpTCP2在不同组织部位的特异性表达发现,该基因在嫩茎、木质部、韧皮部、顶芽中均呈上调表达,且在木质部中表达量最高,表明BpTCP2可能与木质素的生物合成相关。在安家兴等人[11]的研究中,也证明了Class I类PCF亚类的AtTCP11通过改变VND7的表达量从而影响维管束的发育。分析BpTCP2在茎节发育中的表达水平,从第1茎节到第4茎节表达量呈现持续上调的趋势,说明BpTCP2可能参与白桦茎的初期发育,Kieffer等[27]发现了AtTCP15和AtTCP14的突变体通过影响细胞增殖来影响茎节发育。在叶片发育的表达分析中,随着叶龄的增加,BpTCP2表达量整体呈现一个下调的趋势说明BpTCP2可能和延缓衰老相关,张春雷等[28]人在对拟南芥的PCF亚类基因AtTCP15、AtTCP22研究时发现,35S::TCP15-SRDX和35S::TCP22-SRDX同时表现出提前衰老的现象。
近年研究发现TCP转录因子除了在植物发育过程中发挥重要作用外,还参与植物激素及胁迫的信号传导等过程[29],同时我们对BpTCP2基因的启动子序列进行分析,也发现其含有多种激素和胁迫的应答元件(未发表),故本研究对BpTCP2基因进行了外源激素和非生物胁迫应答分析,结果表明,在植物激素处理下,BpTCP2呈现不同的表达趋势。经ABA处理后,BpTCP2呈现出先上调后下调的波动型表达模式,说明该基因参与ABA的信号应答。Mukhopadhyay等[30]对PCF亚类的OsTCP19的信号通路进行研究,发现OsTCP19通过与转录因子ABI4的互作来调控ABI3的表达从而对ABA信号通路进行调控;经BR处理后,BpTCP2整体均呈现下调表达,尤其在4 h时表达量最低,说明BpTCP2明显的响应了BR的信号且为负响应模式。DWARF4是BR生物合成中的关键酶,在拟南芥中,通过染色体免疫共沉淀发现AtTCP1可以促进DWF4的表达,使内源BR含量增加,促进植物的生长[29];在IAA处理后,BpTCP2在6 h时呈现下调表达的趋势而在其他时间点表达量变化并不明显,说明BpTCP2也响应了IAA的初期信号。在拟南芥中CIN类的AtTCP3可以引起IAA合成的负调节因子CYP83B1/SUR2的上调[31],AtTCP15(PCF类)也可间接诱导IAA3/SHY2表达[2],通过使用IAA响应原件DR5::GUS分析,AtTCP15和AtTCP3在一定程度上功能冗余,表明他们在IAA信号通路中可能发挥着相同的作用,这与BpTCP2处理后的结果类似;在JA处理后,BpTCP2除6 h、12 h与对照相比变化不明显外,其他时间点均呈现上调表达的趋势,说明BpTCP2响应了JA的信号且为正响应模式,在冯志娟等人[29]的研究中,LIPOXYGENASE2(LOX2)基因受TCP转录因子的调控且LOX2的诱导使JA积累;在SA处理下,BpTCP2的表达量在12 h前均为上调表达,但在24 h时骤然下降,说明BpTCP2响应了SA的信号,拟南芥AtTCP8(PCF亚类)与ICS1转录激活因子SARD1和WRKY28以及ICS1阻遏蛋白NAC019相互作用,ICS1基因是编码SA生物合成过程中必需的酶[32]且拟南芥中AtTCP8可以作为效应诱导免疫过程中的正向调节者[29]。
在CdCl2、NaCl、NaHCO3处理过程中,BpTCP2呈现上调表达的趋势,表明该基因在相应的非生物胁迫过程中表现出正调控模式,这一结果与水曲柳(Fraxinus mandschurica)和木薯(Manihot esculenta)的研究结果一致,水曲柳的FmTCP4和木薯的MeTCPs基因在盐胁迫下也呈现出正响应模式[33-34];在水稻中,OsTCP19的过表达导致LOX2(茉莉酸信号通路基因)的下调,从而减少水分流失和活性氧的缺失以及脂肪的积累,进而提高植株的耐盐性[30];本实验团队前期对PromTCP7::GUS拟南芥株系进行盐、旱处理,发现该基因对盐、旱途径均有不同程度响应[35]。耐盐性结果与本实验基本一致,但在PEG(干旱)处理下,BpTCP2的变化并不明显。目前,miR319的靶位点已在多个物种的TCP基因中被发现,在植物非生物胁迫应答的过程中,miR319与TCP基因的模式靶向关系为负调控[29],作为miR319的靶基因,TCP家族基因间存在功能冗余与分歧;并且在拟南芥中Viola等[36]发现,半胱氨酸(Cys-20)是AtTCP15保守结构域中的重要组成部分,AtTCP15通过其在氧化胁迫条件下发生的氧化反应从而抑制该基因的转录水平,进而参与非生物胁迫过程。
4. 结 论
本实验成功克隆了BpTCP2基因,生物信息分析表明该基因属于Class I类PCF亚类且含有高度保守的bHLH结构域;BpTCP2参与木质素的生物合成、初期茎节发育及叶片发育过程;BpTCP2参与植物外源激素(ABA、IAA、BR、JA、SA)及非生物胁迫(重金属、盐、碱)的响应。
-
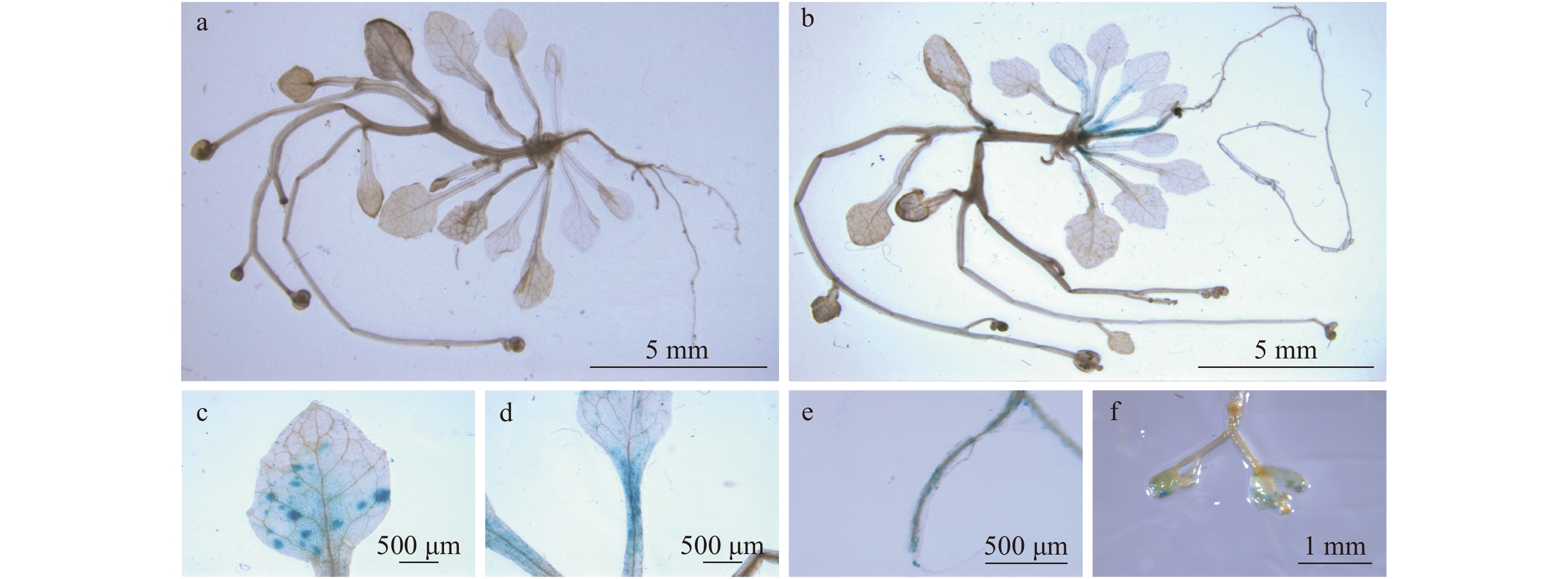
图 1 转ProSPL8::GUS拟南芥的组织化学GUS测定
a. 野生型拟南芥未检测到GUS活性;b. GUS活性在转ProSPL8::GUS拟南芥的下胚轴、叶片、叶柄、根和花序检测到;c. 转ProSPL8::GUS拟南芥叶片;d. 转ProSPL8::GUS拟南芥叶柄;e. 转ProSPL8::GUS拟南芥根;f. 转ProSPL8::GUS拟南芥花序。a, wild type Arabidopsis thaliana without GUS activity; b, GUS activity was observed in hypocotyls, leaves, petioles, roots and inflorescences of ProSPL8::GUS transgenic Arabidopsis thaliana; c, leaves of ProSPL8::GUS; d, petiole of ProSPL8::GUS; e, root of ProSPL8::GUS; f, inflorescence of ProSPL8::GUS.
Figure 1. Histochemical GUS staining of ProSPL8::GUS transgenic Arabidopsis thaliana
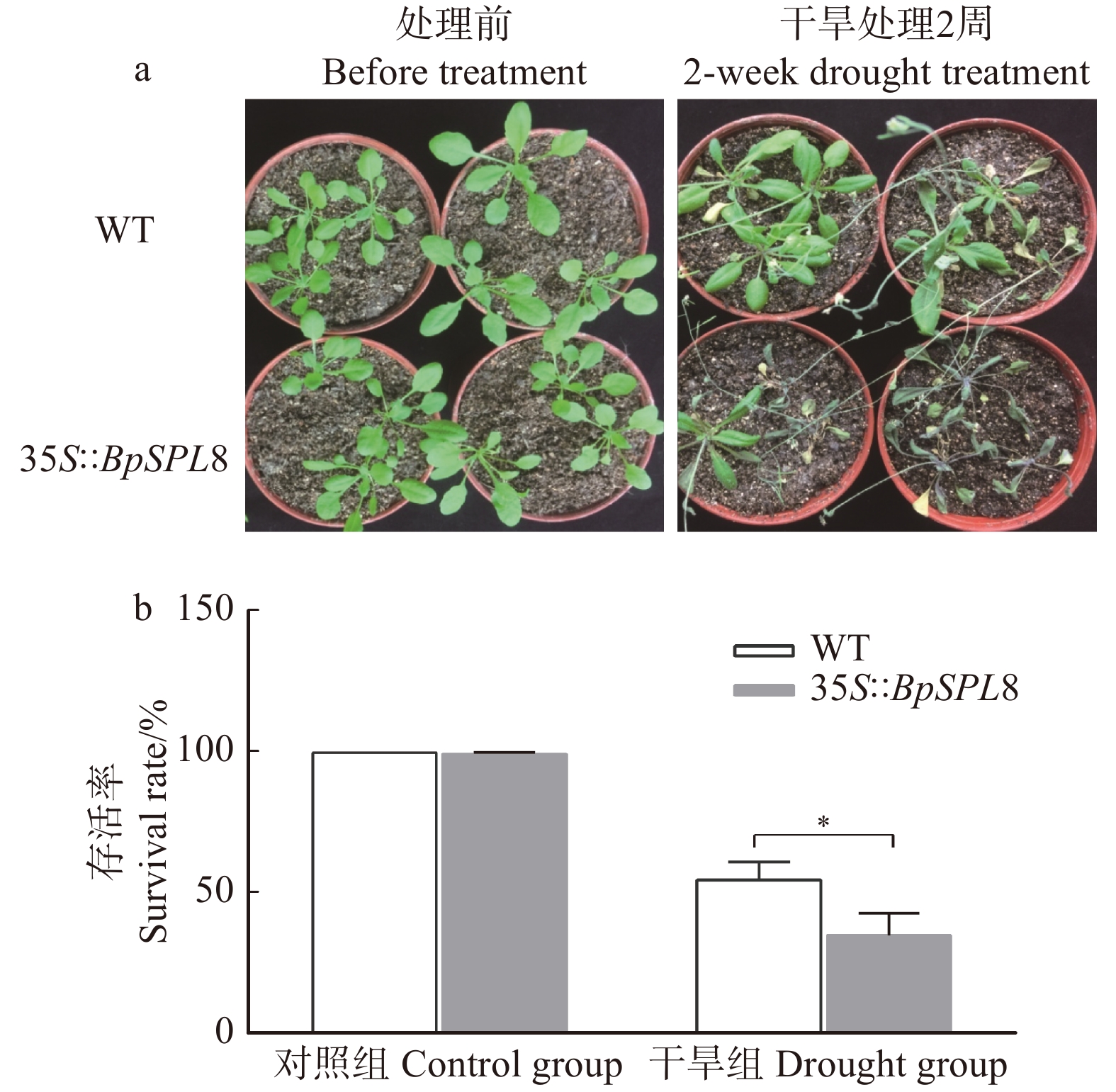
图 3 干旱处理条件下野生型和35S::BpSPL8转基因拟南芥的生长状态及存活率
WT为野生型拟南芥,35S::BpSPL8为BpSPL8过表达拟南芥。a. 处理前和干旱2周后野生型和35S::BpSPL8转基因拟南芥的表型;b. 干旱胁迫2周复水3 d后和正常生长条件下野生型和35S::BpSPL8转基因拟南芥的存活率(*P < 0.05)。WT is wild-type Arabidopsis thaliana, 35S::BpSPL8 is BpSPL8 overexpressing Arabidopsis thaliana. a, phenotype of wild-type and 35S::BpSPL8 transgenic Arabidopsis thaliana before treatment and 2 weeks after drought; b, survival rate of wild-type and 35S::BpSPL8 transgenic Arabidopsis thaliana under normal growth conditions and re-watering 3 days after 2 weeks of drought treatment (*P < 0.05).
Figure 3. Growth status and survival rate of wild-type and 35S::BpSPL8 transgenic Arabidopsis thaliana under drought stress
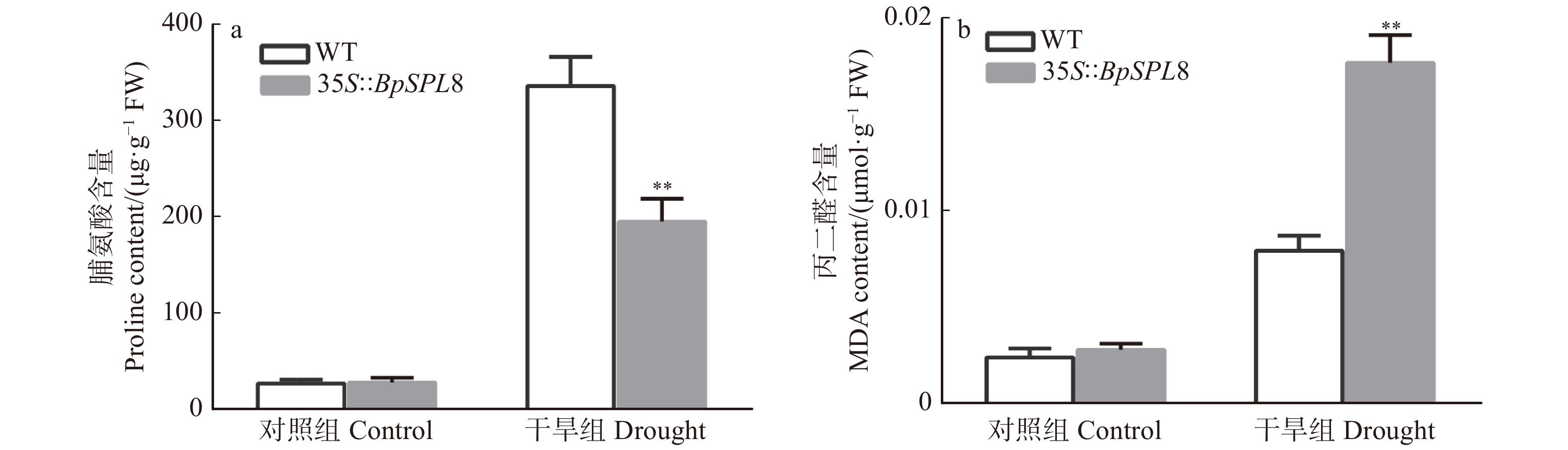
图 4 正常生长和干旱7 d 35S::BpSPL8转基因和野生型拟南芥脯氨酸和丙二醛的含量测定
WT为野生型拟南芥(**P < 0.01),35S::BpSPL8为BpSPL8过表达拟南芥。WT is wild-type Arabidopsis thaliana, 35S::BpSPL8 is BpSPL8 overexpressing Arabidopsis thaliana (**P < 0.01).
Figure 4. Contents of proline and malondialdehyde in wild type and 35S::BpSPL8 transgenic Arabidopsis thaliana under normal growth conditions and 7 days of drought treatment
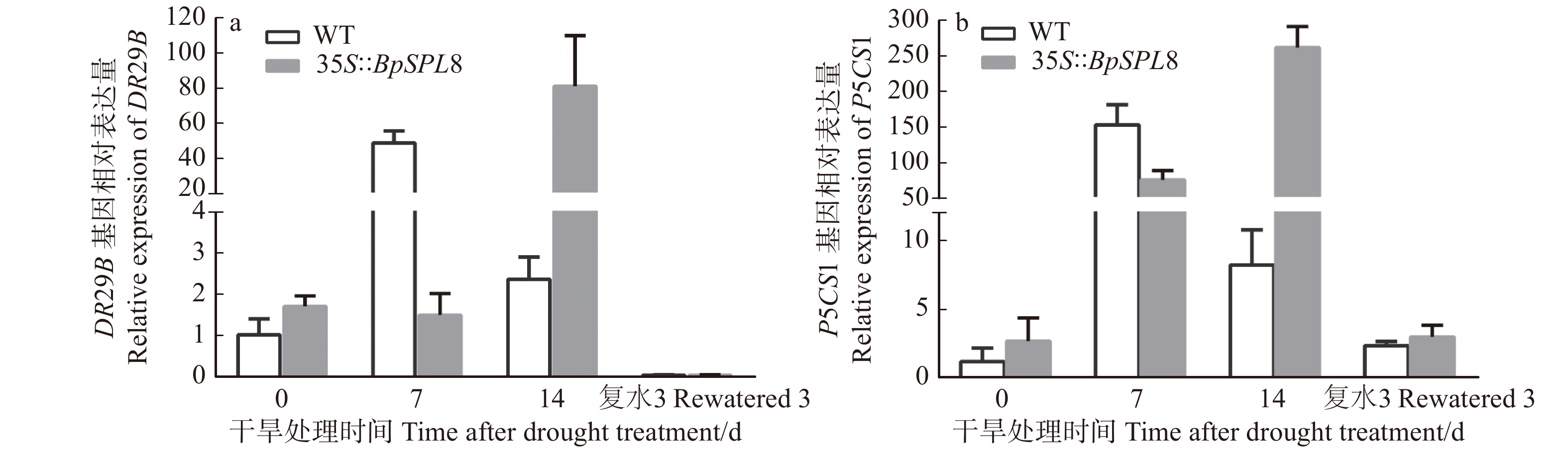
图 5 干旱处理下野生型和35S::BpSPL8转基因拟南芥DR29B和P5CS1表达模式分析
WT为野生型拟南芥;35S::BpSPL8为BpSPL8过表达拟南芥。WT is wild-type Arabidopsis thaliana, 35S::BpSPL8 is BpSPL8 overexpressing Arabidopsis thaliana.
Figure 5. Expression pattern analysis of DR29B and P5CS1 in wild type and
35S::BpSPL8 transgenic Arabidopsis thaliana under drought treatment 表 1 BpSPL8启动子中的顺式作用元件及相关功能预测
Table 1 Cis-acting elements and predicted functions in the sequence of BpSPL8 promoter
顺式元件 Cis-element 数量 Number 功能 Function 功能分类 Function group MBS 4 MYB binding site involved in drought-inducibility Binding site specific response element MRE 1 MYB binding site involved in light responsiveness Binding site specific response element circadian 3 Circadian control Circadian Box-W1 1 Fungal elicitor responsive element Elicitor specific responsive element ABRE 1 Abscisic acid responsiveness Hormone responsive element TGA-element 1 Auxin-responsive element Hormone responsive element GARE-motif 3 Gibberellin-responsive element Hormone responsive element ERE 1 Ethylene-responsive element Hormone responsive element TCA-element 1 Salicylic acid responsiveness Hormone responsive element AE-box 2 Light response Light responsive element Box 4 3 Light response Light responsive element Box I 3 Light responsive element Light responsive element CATT-motif 4 Light responsive element Light responsive element GAG-motif 1 Light responsive element Light responsive element GA-motif 2 Light responsive element Light responsive element GT1-motif 1 Light responsive element Light responsive element H-box 1 Light responsive element Light responsive element Sp1 6 Light responsive element Light responsive element TCT-motif 1 Light responsive element Light responsive element G-Box 2 Light responsiveness Light responsive element G-box 3 Light responsiveness Light responsive element ATGCAAAT motif 1 Associated to the TGAGTCA motif Plant tissue-specific element MSA-like 1 Cell cycle regulation Plant tissue-specific element GCN4_motif 1 Endosperm expression Plant tissue-specific element Skn-1_motif 3 Endosperm expression Plant tissue-specific element CAT-box 1 Meristem expression Plant tissue-specific element AC-II 1 Negative regulation of phloem expression Plant tissue-specific element as-2-box 1 Shoot-specific expression and light responsiveness Plant tissue-specific element ARE 1 Anaerobic response element Stress responsive element TC-rich repeats 1 Defense and stress responsiveness Stress responsive element HSE 2 Heat stress responsiveness Stress responsive element GCC box 1 Wounding and pathogen responsiveness Stress responsive element W box 1 Wounding and pathogen responsiveness Stress responsive element 5UTR Py-rich stretch 2 Conferring high transcription levels Transcription regulation element AAGAA-motif 1 Unknown Unnamed__1 2 Unknown Unnamed__3 2 Unknown Unnamed__4 7 Unknown -
[1] Cardon G, Hohmann S, Klein J, et al. Molecular characterisation of the Arabidopsis SBP-box genes[J]. Gene, 1999, 237(1): 91−104. doi: 10.1016/S0378-1119(99)00308-X
[2] Schwab R, Palatnik J F, Riester M, et al. Specific effects of microRNAs on the plant transcriptome[J]. Developmental Cell, 2005, 8(4): 517−527. doi: 10.1016/j.devcel.2005.01.018
[3] Wu G, Poethig R S. Temporal regulation of shoot development in Arabidopsis thaliana by miR156 and its target SPL3[J]. Development, 2006, 133(18): 3539−3547. doi: 10.1242/dev.02521
[4] Wang J W, Czech B, Weigel D. miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana[J]. Cell, 2009, 138(4): 738−749. doi: 10.1016/j.cell.2009.06.014
[5] Yu N, Cai W J, Wang S, et al. Temporal control of trichome distribution by microRNA156-targeted SPL genes in Arabidopsis thaliana[J]. Plant Cell, 2010, 22(7): 2322−2335. doi: 10.1105/tpc.109.072579
[6] Jung J H, Seo P J, Kang S K, et al. miR172 signals are incorporated into the miR156 signaling pathway at the SPL3/4/5 genes in Arabidopsis developmental transitions[J]. Plant Molecular Biology, 2011, 76(1−2): 35−45. doi: 10.1007/s11103-011-9759-z
[7] Shikata M, Koyama T, Mitsuda N, et al. Arabidopsis SBP-box genes SPL10, SPL11 and SPL2 control morphological change in association with shoot maturation in the reproductive phase[J]. Plant and Cell Physiology, 2009, 50(12): 2133−2145. doi: 10.1093/pcp/pcp148
[8] Schwarz S, Grande A V, Bujdoso N, et al. The microRNA regulated SBP-box genes SPL9 and SPL15 control shoot maturation in Arabidopsis[J]. Plant Molecular Biology, 2008, 67(1−2): 183−195. doi: 10.1007/s11103-008-9310-z
[9] Yamasaki H, Hayashi M, Fukazawa M, et al. SQUAMOSA promoter binding protein-like7 is a central regulator for copper homeostasis in Arabidopsis[J]. Plant Cell, 2009, 21(1): 347−361. doi: 10.1105/tpc.108.060137
[10] Stone J M, Liang X, Nekl E R, et al. Arabidopsis AtSPL14, a plant-specific SBP-domain transcription factor, participates in plant development and sensitivity to fumonisin B1[J]. Plant Journal, 2005, 41(5): 744−754. doi: 10.1111/tpj.2005.41.issue-5
[11] Chao L M, Liu Y Q, Chen D Y, et al. Arabidopsis transcription factors SPL1 and SPL12 confer plant thermotolerance at reproductive stage[J]. Molecular Plant, 2017, 10(5): 735−748. doi: 10.1016/j.molp.2017.03.010
[12] Unte U S, Sorensen A M, Pesaresi P, et al. SPL8, an SBP-box gene that affects pollen sac development in Arabidopsis[J]. Plant Cell, 2003, 15(4): 1009−1019. doi: 10.1105/tpc.010678
[13] Zhang Y, Schwarz S, Saedler H, et al. SPL8, a local regulator in a subset of gibberellin-mediated developmental processes in Arabidopsis[J]. Plant Molecular Biology, 2007, 63(3): 429−439. doi: 10.1007/s11103-006-9099-6
[14] Xing S, Salinas M, Hohmann S, et al. miR156-targeted and nontargeted SBP-box transcription factors act in concert to secure male fertility in Arabidopsis[J]. Plant Cell, 2010, 22(12): 3935−3950. doi: 10.1105/tpc.110.079343
[15] Gou J, Debnath S, Sun L, et al. From model to crop: functional characterization of SPL8 in M. truncatula led to genetic improvement of biomass yield and abiotic stress tolerance in alfalfa[J]. Plant Biotechnology Journal, 2018, 16(4): 951−962. doi: 10.1111/pbi.2018.16.issue-4
[16] 李双, 苏艳艳, 王厚领, 等. 胡杨miR1444b在拟南芥中正调控植物抗旱性[J]. 北京林业大学学报, 2018, 40(4):1−9. Li S, Su Y Y, Wang H L, et al. Populus euphratica miR1444b positively regulates plants response to drought stress in Arabidopsis thaliana[J]. Journal of Beijing Forestry University, 2018, 40(4): 1−9.
[17] 姚琨, 练从龙, 王菁菁, 等. 胡杨PePEX11基因参与调节盐胁迫下拟南芥的抗氧化能力[J]. 北京林业大学学报, 2018, 40(5):19−28. Yao K, Lian C L, Wang J J, et al. PePEX11 functions in regulating antioxidant capacity of Arabidopsis thaliana under salt stress[J]. Journal of Beijing Forestry University, 2018, 40(5): 19−28.
[18] Sakuma Y, Maruyama K, Osakabe Y, et al. Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression[J]. Plant Cell, 2006, 18(5): 1292−1309. doi: 10.1105/tpc.105.035881
[19] Strizhov N, Abraham E, Okresz L, et al. Differential expression of two P5CS genes controlling proline accumulation during salt-stress requires ABA and is regulated by ABA1, ABI1 and AXR2 in Arabidopsis[J]. Plant Journal, 1997, 12(3): 557−569.
[20] 李蕾蕾, 孙丰坤, 李天宇, 等. 白桦BpGT14基因启动子克隆及表达活性分析[J]. 北京林业大学学报, 2016, 38(7):16−24. Li L L, Sun F K, Li T Y, et al. Cloning and activity analysis of BpGT14 gene promoter in Betula platyphylla[J]. Journal of Beijing Forestry University, 2016, 38(7): 16−24.
[21] 张一南, 王洋, 张会龙, 等. 过表达胡杨PeRIN4基因拟南芥提高质膜H+-ATPase活性和耐盐性[J]. 北京林业大学学报, 2017, 39(11):1−8. Zhang Y N, Wang Y, Zhang H L, et al. Overexpression of PeRIN4 enhanced salinity tolerance through up regulation of PM H+-ATPase in Arabidopsis thaliana[J]. Journal of Beijing Forestry University, 2017, 39(11): 1−8.
[22] Jefferson R A, Kavanagh T A, Bevan M W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants[J]. EMBO Journal, 1987, 6(13): 3901−3907. doi: 10.1002/embj.1987.6.issue-13
[23] Liu C, Guan M X, Hu X Q, et al. Complex regulatory network of Betula BplSPL8 in planta[J]. Journal of Forestry Research, 2017, 28(5): 881−889. doi: 10.1007/s11676-017-0372-0
[24] Yu X, Liu Y, Wang S, et al. CarNAC4, a NAC-type chickpea transcription factor conferring enhanced drought and salt stress tolerances in Arabidopsis[J]. Plant Cell Reports, 2016, 35(3): 613−627. doi: 10.1007/s00299-015-1907-5
[25] 牛素贞, 宋勤飞, 樊卫国, 等. 干旱胁迫对喀斯特地区野生茶树幼苗生理特性及根系生长的影响[J]. 生态学报, 2017, 37(21):7333−7341. Niu S Z, Song Q F, Fan W G, et al. Effects of drought stress on leaf physiological characteristics and root growth of the clone seedlings of wild tea plants[J]. Acta Ecologica Sinica, 2017, 37(21): 7333−7341.
[26] Yu N, Niu Q W, Ng K H, et al. The role of miR156/SPLs modules in Arabidopsis lateral root development[J]. Plant Journal, 2015, 83(4): 673−685. doi: 10.1111/tpj.12919
[27] Gao R, Wang Y, Gruber M Y, et al. miR156/SPL10 modulates lateral root development, branching and leaf morphology in Arabidopsis by silencing AGAMOUS-LIKE 79[J/OL]. Frontiers in Plant Science, 2017, 8 (2017−01−04) [2018−06−20]. https://doi.org/10.3389/fpls.2017.02226.
[28] 刘闯. 18个白桦SPLs基因的鉴定及BpSPL8基因的功能分析[D]. 哈尔滨: 东北林业大学, 2017. Liu C. Identification of 18 SPL gene family members and functional analysis of BpSPL8 in Betula platyphylla[D]. Harbin: Northeast Forestry University, 2017.
[29] Saini S, Sharma I, Kaur N, et al. Auxin: a master regulator in plant root development[J]. Plant Cell Reports, 2013, 32(6): 741−757. doi: 10.1007/s00299-013-1430-5
[30] Hao Y J, Wei W, Song Q X, et al. Soybean NAC transcription factors promote abiotic stress tolerance and lateral root formation in transgenic plants[J]. Plant Journal, 2011, 68(2): 302−313. doi: 10.1111/j.1365-313X.2011.04687.x
[31] Chen D, Richardson T, Chai S, et al. Drought-up-regulated TaNAC69-1 is a transcriptional repressor of TaSHY2 and TaIAA7, and enhances root length and biomass in wheat[J]. Plant and Cell Physiology, 2016, 57(10): 2076−2090. doi: 10.1093/pcp/pcw126
[32] Laplaze L, Benkova E, Casimiro I, et al. Cytokinins act directly on lateral root founder cells to inhibit root initiation[J]. Plant Cell, 2007, 19(12): 3889−3900. doi: 10.1105/tpc.107.055863
[33] Loutfy N, El-Tayeb M A, Hassanen A M, et al. Changes in the water status and osmotic solute contents in response to drought and salicylic acid treatments in four different cultivars of wheat (Triticum aestivum)[J]. Journal of Plant Research, 2012, 125(1): 173−184. doi: 10.1007/s10265-011-0419-9
[34] Ivanchenko M G, Muday G K, Dubrovsky J G. Ethylene-auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana[J]. Plant Journal, 2008, 55(2): 335−347. doi: 10.1111/tpj.2008.55.issue-2
[35] Ruiz-Lozano J M, Aroca R, Zamarreno A M, et al. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato[J]. Plant Cell and Environment, 2016, 39(2): 441−452. doi: 10.1111/pce.v39.2
[36] Sanchez-Romera B, Ruiz-Lozano J M, Zamarreno A M, et al. Arbuscular mycorrhizal symbiosis and methyl jasmonate avoid the inhibition of root hydraulic conductivity caused by drought[J]. Mycorrhiza, 2016, 26(2): 111−122. doi: 10.1007/s00572-015-0650-7
[37] Rowe J H, Topping J F, Liu J, et al. Abscisic acid regulates root growth under osmotic stress conditions via an interacting hormonal network with cytokinin, ethylene and auxin[J]. New Phytologist, 2016, 211(1): 225−239. doi: 10.1111/nph.13882
[38] 赵婉莹, 于太飞, 杨军峰, 等. 大豆GmbZIP16的抗旱功能验证及分析[J]. 中国农业科学, 2018, 51(15):6−18. Zhao W Y, Yu T F, Yang J F, et al. Verification and analyses of soybean GmbZIP16 gene resistance to drought[J]. Scientia Agricultura Sinica, 2018, 51(15): 6−18.
-
期刊类型引用(2)
1. 魏红洋,张一帆,董灵波,刘兆刚,陈莹. 帽儿山主要林分类型空间结构状态综合评价. 中南林业科技大学学报. 2021(10): 131-139 .  百度学术
百度学术
2. 刘文桢,袁一超,张连金,赵中华. 基于林分内部状态与邻域环境的油松林稳定性评价. 林业科学. 2021(09): 76-86 .  百度学术
百度学术
其他类型引用(3)



 下载:
下载: