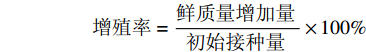Enhancement of embryogenic callus proliferation in Chinese pine (Pinus tabuliformis) by airlift bioreactor
-
摘要:目的大量具有活性的胚性愈伤组织的增殖可为油松体细胞胚胎发生研究和植株再生提供充足的材料,锥形瓶悬浮培养体系下胚性愈伤组织的增殖率低且易酸化坏死,建立气动生物反应器油松胚性愈伤增殖体系可促进油松胚性愈伤组织的增殖。方法本研究以油松愈伤组织为材料,利用L9(34)正交试验设计,探究了生物反应器中愈伤接种量、新旧培养基的配比和激素浓度3个因素对愈伤组织增殖的影响,建立了增殖体系。而后在相同条件下与锥形瓶悬浮培养进行对比。结果结果表明:气动生物反应器中每100 mL液体培养基内接种胚性愈伤10 g,2,4-D 0.2 mg/L,保留20%的旧培养基时能使胚性愈伤增殖率达到最高,可达216.18%;结论与锥形瓶悬浮培养体系相比,ALB系统在一周内胚性愈伤生长速度更快,为锥形瓶悬浮培养体系的3.15倍;显微观察显示增殖的胚性愈伤组织稳定且优质。该研究为基于油松体胚体系的大规模扩繁提供技术支持。Abstract:ObjectiveThe proliferation of a great number of active embryogenic callus can provide sufficient materials for somatic embryogenesis and plant regeneration of Pinus tabuliformis. However, the yield of flask suspension proliferation is limited and easy acidification necrosis. The establishment of airlift bioreactor system for embryogenic callus multiplication of P. tabuliformis can promote the proliferation of embryogenic callus of P. tabuliformis.MethodIn this study, P. tabuliformis callus was used as material, and L9 (34) orthogonal design was used to investigate the effects of three factors on callus proliferation in bioreactor, including inoculation volume, ratio of old and new media and hormone concentration. Then the suspension culture in conical flask was compared with that in conical flask under the same conditions.ResultThe highest embryogenic callus proliferation rate, 216.18% was obtained using 10 g embryogenic callus, 0.2 mg/L 2,4-D and 20% old culture medium inoculated in 100 mL liquid medium in the ALB.ConclusionCompared with conical flask suspension culture, the growth rate of embryogenic callus in ALB system was 2.15 times faster in a week. Microscopic observation shows that the proliferated embryogenic callus is stable and high quality. This study provides technical support for large-scale propagation of P. tabuliformis based on somatic embryo system.
-
Keywords:
- Pinus tabuliformis /
- bioreactor /
- embryogenic callus
-
油松(Pinus tabuliformis)是中国特有的树种,为华北地区最主要的造林树种之一[1],油松耐寒、耐旱、耐土壤瘠薄,在中国北方荒山造林中占有特殊地位,是重要的生态树种和用材树种。但目前传统的繁育方式如嫁接、种子繁殖等都面临着许多问题,例如生长周期长、有性繁殖后代变异较大、扦插等无性繁殖生根率低、对一些优良性状难以维持,不能满足试验和生产的需要[2]。
体细胞胚胎发生(Somatic embryogenesis)是指在体外培养的条件下,植物组织不经过受精,却又与合子胚发育进程相似,形成具有双极性胚性结构的过程[3],是目前林木上最具潜力的繁殖手段[4]。这种体外营养繁殖方式最先在胡萝卜上进行[5],随着生物技术的进一步发展,体胚发生已经在被子植物和裸子植物中被广泛应用。针叶树体胚发生的报道最早见于班克松,随着研究的不断开展,目前已在欧洲赤松等多个针叶树获得体细胞胚[6-7]。
油松体胚体系的研究刚刚起步,胚性愈伤组织虽然已经被成功诱导[8],但仍然存在很多技术环节的缺陷;锥形瓶悬浮培养虽然能供应均匀的细胞材料[9],较半固体增殖速度快,但这种悬浮培养体系的产量依然不能满足生产和实验的大量需要[10],且时常有组织褐化坏死发生。Kong等[11]的气动生物反应器(Airlift bioreactors, ALB)是一种新型的增殖培养方式,ALB系统主要包括3个部分:第一个是供气部分(汽泵),第二个是输气和控制部分(管道与汽阀),第三个是单个或多个生物反应器。空气进入生物反应器,在液体培养物中产生许多小气泡,这些气泡既搅动胚胎细胞团,防止材料堆积,又向培养物提供空气。相较于悬浮培养方式,生物反应器具有很多优点:如工作体积小、单位体积生产能力高、物理和化学条件控制方便等[12],而ALB除以上优点外,还具有可靠、易于操作安装、经济实用等特点,适合实验室环境的应用[13]。
针叶树体细胞原胚较大、易下沉、胚柄长、易结团,应用ALB系统时需要考虑接种量、旧培养基的保留等重要因素对培养过程的影响[11],其效果也需经过进一步的检验。因此,我们基于已建立的油松胚性愈伤锥形瓶悬浮培养体系,利用ALB按照正交试验设计研究了不同初始接种量、新旧培养基配比和激素浓度组合对油松胚性愈伤增殖的影响,选出最优组合,并通过与锥形瓶悬浮培养的增殖量对比及胚性愈伤形态观察来检验其效果,最终建立高效可靠的增殖体系。
1. 材料与方法
1.1 材 料
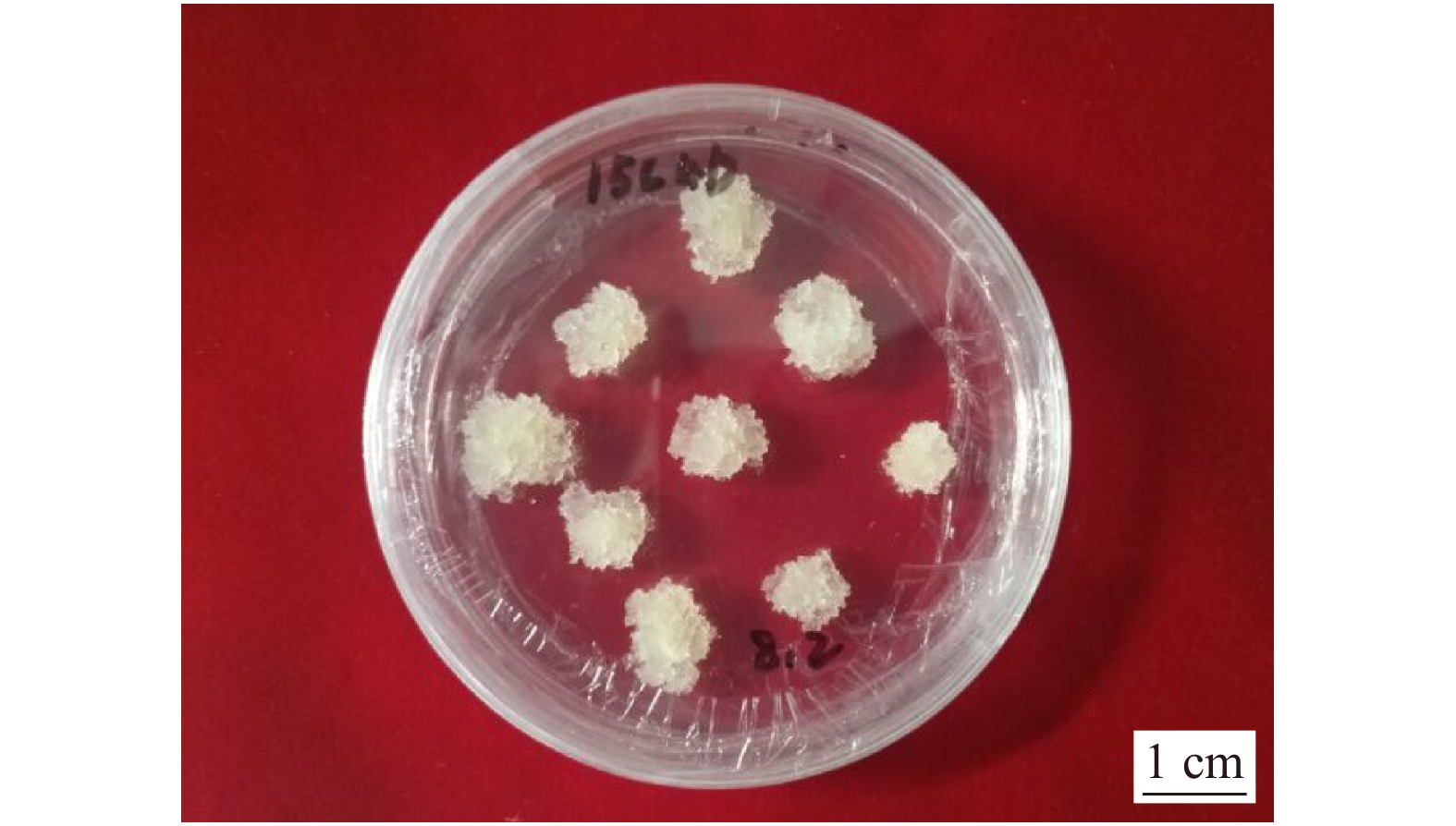
油松ALB增殖体系建立和锥形瓶悬浮培养所需的试验材料均为2015年获得的胚性细胞系15c4b(图1)[14]。探究不同继代年龄的胚性细胞系与增殖率关系的试验材料分别是继代年龄为3年、2年、1年及0.25年的胚性细胞系,每个细胞系各1个。
1.2 方 法
1.2.1 液体培养基
采用mLV[15]为基本培养基,mLV为油松愈伤组织增殖培养基,根据前人有关针叶树体胚发生的研究经过本实验室改良后得出的培养基配方,添加蔗糖20 g/L,酶水解酪蛋白500 mg/L,不添加植物凝胶,pH为5.8,121 ℃灭菌15 min,因谷氨酰胺不耐高温,所以当液体培养基温度降至50 ℃以下时,将500 mg/L过滤灭菌的谷氨酰胺加入其中。
1.2.2 反应体系试验方法
基于已建立的锥形瓶悬浮培养体系(每80 mL液体培养基内接种胚性愈伤10 g,添加0.1 mg/L 2,4-D,培养温度25 ℃)对生物反应器中影响胚性愈伤增殖的因素(每100 mL液体培养基的胚性愈伤组织接种量、旧培养基保留量和激素浓度)按正交试验设计开展试验,试验共设9个处理,每个处理重复3次,样本量分别为5、10、15 g,总样本量为240 g,具体如正交试验设计(表1)所示,黑暗培养1周后统计增殖率。
表 1 正交设计表L9(34)Table 1. Orthogonal design L9 (34)编号
No.接种量
Inoculum size/g2,4-D质量浓度
2,4-D mass concentration/
(mg·L− 1)旧培养基保留量
Retention of old medium/mL1 5 0.1 20 2 5 0.2 50 3 5 0.3 80 4 10 0.1 50 5 10 0.2 80 6 10 0.3 20 7 15 0.1 80 8 15 0.2 20 9 15 0.3 50 增殖率=鲜质量增加量初始接种量×100% 1.2.3 ALB体系与锥形瓶悬浮培养体系的胚性愈伤生长量对比
采用正交试验最优反应体系,统计ALB增殖体系下油松胚性愈伤1周内的生长量情况,并与锥形瓶悬浮培养方式中的愈伤生长量对比,锥形瓶悬浮培养条件为每80 mL液体培养基内接种胚性愈伤10 g,2,4-D为0.1 mg/L,无旧培养基保留。两种培养方式下的每个处理均设置3次重复,锥形瓶悬浮培养每次重复的样本量为10 g,总样本量为30 g,ALB增殖条件下样本量为正交试验最优接种量。
1.2.4 ALB体系与锥形瓶悬浮培养胚性愈伤pH值的对比
依照上述最优的正交设计反应体系,对ALB体系与锥形瓶悬浮培养体系中的胚性愈伤增殖过程中pH值的变化进行对比试验,两种培养方式下每个处理均设置3次重复,样本量同上。黑暗条件下培养,每隔1天统计pH值。
1.2.5 不同继代年龄的胚性愈伤与增殖率的关系
依照上述最优的正交设计反应体系,将2015至2018年不同继代年龄的4种油松胚性愈伤,转入生物反应器中,对油松胚性愈伤的增殖继代年龄与增殖率的关系进行探究。4种不同继代年龄的愈伤组织,共4个处理,每个处理均设置3次重复,样本量为正交试验最优接种量。黑暗条件下培养1周后统计增殖率。
1.3 油松愈伤组织胚性结构形态检验
取少量胚性愈伤组织放在载玻片上,用解剖镜(Olympus SZ)观察外表形态;另取微量愈伤组织放置于载玻片上,用卡宝品红(Leagene DZ0040)染色4 min后盖上盖玻片[16],在显微镜(Olympus BX43)下观察胚性愈伤组织的胚性结构,随机取样并重复3次。
1.4 数据分析
试验数据用Microsoft Excel 2016和SPSS 19.0软件进行处理分析,利用最小显著差数法(LSD)进行多重比较。
2. 结果与分析
2.1 正交试验结果
ALB条件下,油松胚性愈伤增殖率的正交试验结果见表2。9组处理中6号和8号处理的增殖率高于200%,分别达到206.87%和216.18%;9号增殖率超过150%,为169.56%;1号、2号、4号增殖率在100%以上,分别为139.67%、143.33%和111.00%;3号为58.93%,7号增值率最低,为11.80%。极差分析显示,本试验选定的最优组合为每100 mL液体培养基内接种胚性愈伤10 g,2,4-D 0.2 mg/L,旧培养基保留量20 mL。通过极差分析,发现旧培养基保留量对结果影响最大。方差分析结果显示,3个因素均对愈伤组织增殖量有显著影响(表3)。
表 2 接种量、激素浓度、旧培养基保留量对愈伤组织增殖量的影响Table 2. Effects of inoculation quantity, hormone concentrations and the ratio of used medium on growth rate编号
No.接种量
Inoculum size/g2,4-D质量浓度
2,4-D mass concentration/(mg·L− 1)旧培养基保留量
Retention of old medium/mL增殖量
Proliferation/g1 5 0.1 20 139.67 ± 18.21 2 5 0.2 50 143.33 ± 14.70 3 5 0.3 80 58.93 ± 6.52 4 10 0.1 50 111.0 ± 10.91 5 10 0.2 80 96.3 ± 4.03 6 10 0.3 20 206.87 ± 8.29 7 15 0.1 80 11.80 ± 4.75 8 15 0.2 20 216.18 ± 5.00 9 15 0.3 50 169.56 ± 7.16 k1 113.98 87.49 187.57 k2 138.06 151.94 141.30 k3 132.51 145.12 55.68 R 24.08 64.45 131.89 最优组合 Optimal combination 10 0.2 20 注:表中数据为平均值 ± 标准差。Note: the data in the table are mean ± standard deviation. 表 3 ALB试验方差分析Table 3. Analysis of variance of ALB test变异来源 Source of variation 自由度 df 均方 MS F 值 F value 显著性 Sig. 接种量 Inoculum size 2 1 427.125 153.380 0.042* 旧培养基保留量 Retention of old medium 2 318.063 34.184 0.031* 2,4-D质量浓度 2,4-D mass concentration 2 553.212 59.456 0.027* 误差 Error 20 9.304 总变异 Total variation 26 注:*表示在P < 0.05水平差异显著。Note: * represents significant difference at P < 0.05 level. 2.2 ALB体系与锥形瓶悬浮培养的胚性愈伤生长量对比
为了验证优化培养基的增殖效果,将各因素最佳水平进行组合,即采用每100 mL液体培养基内接种胚性愈伤10 g,2,4-D质量浓度为0.2 mg/L,保留20 mL旧培养基的组合,与锥形瓶悬浮培养的增殖量进行对比。从图2可以看出随着培养时间的增加,胚性愈伤增殖量也逐渐增加。第1天时,两种培养方式的增殖量并没有差异,但从第2天开始,生物反应器与锥形瓶悬浮培养开始出现显著差异,到第7天时增殖量相差24.95 g,较原培养基多产生3.15倍的愈伤组织。所以,与原培养基相比,优化培养基的增殖效果是比较显著的。同时,愈伤组织增殖稳定、健康,一定程度上说明生物反应器增殖方式适合愈伤组织增殖培养。
2.3 ALB体系与锥形瓶悬浮培养胚性愈伤pH值的对比
如图3所示,从第1天到第7天,锥形瓶培养的愈伤组织与生物反应器培养的愈伤组织pH值差异不明显,但从第8天开始,锥形瓶愈伤组织的pH值明显降低,生物反应器内愈伤组织pH值虽有下降趋势,但与锥形瓶相比下降缓慢。到第14天时,锥形瓶内的pH值已经降至3.61,根据已有的研究[14],pH值为5.8时,最利于胚性愈伤组织增殖,此时的环境已经不利于愈伤组织的增殖。培养基pH值过高或过低都不利于愈伤组织的生长,与常规的锥形瓶悬浮培养相比,生物反应器培养方式显然更利于愈伤组织的生长。
2.4 不同继代年龄的胚性愈伤与增殖率的关系
在测试的基因型间,胚性愈伤的继代年龄是不同的,由图4可知,经过相关性的分析,发现增殖继代周期越长,增殖率越低,它们之间呈负相关线性关系,相关系数为− 0.972,在0.01水平上显著相关(P < 0.001),即随着增殖继代年龄的增加,增殖率会降低。
2.5 油松愈伤组织胚性结构形态检验
两种培养方式培养的胚性愈伤组织如图5A和5B所示,显微镜观察显示胚性愈伤保留有明显的胚头、胚柄结构(图5C),表明通入的气体未将胚性愈伤特有的胚性结构损坏,而锥形瓶悬浮培养的愈伤组织观察不到典型的胚胎结构(图5D)。综上所述,在ALB系统内进行胚性愈伤培养可以保持其结构的完整性。
![]() 图 5 锥形瓶悬浮培养和生物反应器培养的愈伤组织形态对比A. ALB培养的胚性愈伤组织;B. 锥形瓶悬浮培养的胚性愈伤组织;C. 体式显微镜下观察到的ALB培养的胚性愈伤组织结构,黄色和红色箭头分别表示胚柄和胚头;D. 体式显微镜下观察到锥形瓶悬浮培养的胚性愈伤组织结构。A, embryogenic callus cultured by ALB; B, embryogenic callus suspension culture in conical flask; C, embryogenic callus structure in ALB observed under the stereo microscope; yellow and red arrows represent the suspensor and the blastocephalon, respectively; D, embryogenic callus in suspension culture observed under the stereo microscope.Figure 5. Comparison of callus morphology between flask suspension culture and bioreactor culture
图 5 锥形瓶悬浮培养和生物反应器培养的愈伤组织形态对比A. ALB培养的胚性愈伤组织;B. 锥形瓶悬浮培养的胚性愈伤组织;C. 体式显微镜下观察到的ALB培养的胚性愈伤组织结构,黄色和红色箭头分别表示胚柄和胚头;D. 体式显微镜下观察到锥形瓶悬浮培养的胚性愈伤组织结构。A, embryogenic callus cultured by ALB; B, embryogenic callus suspension culture in conical flask; C, embryogenic callus structure in ALB observed under the stereo microscope; yellow and red arrows represent the suspensor and the blastocephalon, respectively; D, embryogenic callus in suspension culture observed under the stereo microscope.Figure 5. Comparison of callus morphology between flask suspension culture and bioreactor culture3. 讨 论
相较于锥形瓶悬浮培养,ALB系统能显著提高愈伤组织的增殖量,为油松体胚的大规模生产提供技术支持。同时,ALB系统能将新鲜的空气源源不断的补充到培养基里,保证愈伤组织的正常呼吸,因而更有利于其生长[17]。
悬浮细胞的生长具有群聚效应,细胞密度过低,悬浮细胞生长缓慢,但细胞密度过高时,细胞生长速度过快,细胞液泡变大,悬浮细胞容易积累有害物质,不利于悬浮细胞系的建立[18]。因此,许多植物悬浮培养的研究都把初始接种量当做最重要因素来研究[19]。本试验的结果表明,添加适宜比例的旧培养基有利于油松胚性愈伤组织的增殖。在墨西哥甜玉米的悬浮培养期间,当悬浮培养液在旧培养基[20]存在的情况下,与全新的培养基相比,原生质体形成增加。旧培养基中可能含有利于细胞增殖的物质,新培养基中添加适量使用过的原培养基有利于细胞的适应和生长[21]。并且从实际的角度来看,保留使用过的培养基也可以减轻工作量,对愈伤组织的生长有一定的促进作用[22-23]。
本研究的结果显示,ALB培养比悬浮培养多产生3.15倍的愈伤组织(如图2所示)。Kong等[11]利用生物反应器对美洲板栗开展了胚性愈伤组织增殖的研究,结果表明,与传统的悬浮培养相比,生物反应器培养愈伤增殖量显著提高;Jay等[24]基于生物反应器对胡萝卜细胞进行增殖培养,研究表明,在适宜的培养条件下胡萝卜细胞的增重明显;Tapia等[25]利用生物反应器对葡萄胚性愈伤组织进行培养,结果发现在整个增殖过程中愈伤生长量呈现指数增长。以上研究与本研究结果共同说明生物反应器是提高愈伤增殖量的有效培养方式。
利用生物反应器不仅能够快速、大量获得试验材料,而且能够保证材料正常生长发育。本研究基于ALB系统获得了结构完整的胚性愈伤组织。李玲莉等[13]以香水百合的鳞片愈伤组织为材料进行生物反应器的培养研究,试验结果表明该方式更利于百合鳞片愈伤组织的正常生长;Kong等[26]基于生物反应器系统对美洲板栗胚性愈伤组织进行研究,获得了大小均一、易分散且能够持续生长的细胞,可以作为遗传转化的优良材料。
植物细胞易于粘附成团,形成聚集体[27],而单一依靠摇床的震荡力,在培养后期很有可能无法将组织摇散,导致细胞堆积,产生各种不利于胚性愈伤增殖的因素。鼓泡式生物反应器将空气通到瓶底,从而使细胞均一分散到液体培养基中,同时也减少了胚性愈伤因无氧呼吸产物过量积累而褐化坏死的情况,维持了细胞活性,从而提高了胚性愈伤组织的质量。本研究发现,在愈伤组织增殖过程中,生物反应器的pH值会有一定程度下降,造成这种现象的原因可能是组织内部产生的酸类物质释放到液体培养基中,引起pH值下降;另一方面培养基水分的蒸发也会造成培养基pH值下降。同时我们注意到,生物反应器pH值下降趋势与锥形瓶悬浮培养相比更为缓慢,有效减缓了培养周期中pH值下降过快对愈伤增殖产生的不利影响。此外,油松愈伤增殖率随继代年龄的增加呈明显线性下降趋势。同样发现,油松愈伤组织因长期继代培养会造成胚性能力的下降甚至丧失[10]。
本研究首次利用ALB系统建立了油松胚性愈伤组织的高效增殖体系。该方法成本低、组装操作简单、所需空间环境小、胚性愈伤增殖量和品质显著优于锥形瓶悬浮培养,未来可以用于油松的大规模扩繁以及遗传传化研究。
-
图 5 锥形瓶悬浮培养和生物反应器培养的愈伤组织形态对比
A. ALB培养的胚性愈伤组织;B. 锥形瓶悬浮培养的胚性愈伤组织;C. 体式显微镜下观察到的ALB培养的胚性愈伤组织结构,黄色和红色箭头分别表示胚柄和胚头;D. 体式显微镜下观察到锥形瓶悬浮培养的胚性愈伤组织结构。A, embryogenic callus cultured by ALB; B, embryogenic callus suspension culture in conical flask; C, embryogenic callus structure in ALB observed under the stereo microscope; yellow and red arrows represent the suspensor and the blastocephalon, respectively; D, embryogenic callus in suspension culture observed under the stereo microscope.
Figure 5. Comparison of callus morphology between flask suspension culture and bioreactor culture
表 1 正交设计表L9(34)
Table 1 Orthogonal design L9 (34)
编号
No.接种量
Inoculum size/g2,4-D质量浓度
2,4-D mass concentration/
(mg·L− 1)旧培养基保留量
Retention of old medium/mL1 5 0.1 20 2 5 0.2 50 3 5 0.3 80 4 10 0.1 50 5 10 0.2 80 6 10 0.3 20 7 15 0.1 80 8 15 0.2 20 9 15 0.3 50 表 2 接种量、激素浓度、旧培养基保留量对愈伤组织增殖量的影响
Table 2 Effects of inoculation quantity, hormone concentrations and the ratio of used medium on growth rate
编号
No.接种量
Inoculum size/g2,4-D质量浓度
2,4-D mass concentration/(mg·L− 1)旧培养基保留量
Retention of old medium/mL增殖量
Proliferation/g1 5 0.1 20 139.67 ± 18.21 2 5 0.2 50 143.33 ± 14.70 3 5 0.3 80 58.93 ± 6.52 4 10 0.1 50 111.0 ± 10.91 5 10 0.2 80 96.3 ± 4.03 6 10 0.3 20 206.87 ± 8.29 7 15 0.1 80 11.80 ± 4.75 8 15 0.2 20 216.18 ± 5.00 9 15 0.3 50 169.56 ± 7.16 k1 113.98 87.49 187.57 k2 138.06 151.94 141.30 k3 132.51 145.12 55.68 R 24.08 64.45 131.89 最优组合 Optimal combination 10 0.2 20 注:表中数据为平均值 ± 标准差。Note: the data in the table are mean ± standard deviation. 表 3 ALB试验方差分析
Table 3 Analysis of variance of ALB test
变异来源 Source of variation 自由度 df 均方 MS F 值 F value 显著性 Sig. 接种量 Inoculum size 2 1 427.125 153.380 0.042* 旧培养基保留量 Retention of old medium 2 318.063 34.184 0.031* 2,4-D质量浓度 2,4-D mass concentration 2 553.212 59.456 0.027* 误差 Error 20 9.304 总变异 Total variation 26 注:*表示在P < 0.05水平差异显著。Note: * represents significant difference at P < 0.05 level. -
[1] 齐力旺, 杨云龙, 韩素英, 等. 油松封顶芽的组织培养[J]. 植物生理学通讯, 1995, 31(1):40−44. Qi L W, Yang Y L, Han S Y, et al. Tissue culture of dormant buds from Pinus tabulaeformis[J]. Plant Physiology Communications, 1995, 31(1): 40−44.
[2] 张成高. 油松和美国黄松无性繁殖技术研究[D]. 杨凌: 西北农林科技大学, 2005. Zhang C G. Study on asexual propagation technology of Pinus tabulaeformis and Pinus ponderosa[D]. Yangling: Northwest A&F University, 2005.
[3] 万婷. 油松胚性愈伤组织诱导研究[D]. 太原: 山西大学, 2010. Wan T. Induction of embryogenic callus of Chinese pine (Pinus tabulaeformis C.)[D]. Taiyuan: Shanxi University, 2010.
[4] 韩珊. 红叶乌桕组织培养及植株再生研究[D]. 成都: 四川农业大学, 2006. Han S. Study on tissue culture and plant regeneration of euphorbia cotinifocie Linn[D]. Chengdu: Sichuan Agricultural University, 2006.
[5] Steward F, Mapes M, Mears K. Growth and organized development of cultured cells (Ⅱ): organization in cultures grown from freely suspended cells[J]. American Journal of Botany, 1958, 45: 705−708. doi: 10.1002/j.1537-2197.1958.tb10599.x
[6] Lelu-Water M A, Bernier-Cardou M, Klimaszewska K. Simplified and improved somatic embryogeneis for clonal propagation of Pinus pinaster (Ait.)[J]. Plant Cell Report, 2006, 25(8): 767−776. doi: 10.1007/s00299-006-0115-8
[7] Jain S M, Gupta P K, Newton R J. Protocol for somatic embryogenesis in woody plants[M]. New York : Springer-Verlag, 2005:114−127.
[8] 张金凤, 李慧, 赵健, 等. 一种油松体细胞发生与植株再生方法: CN201410573714.1[P]. 中国专利, 2016. Zhang J F, Li H, Zhao J, et al. Somatic embryogenesis and plant regeneration of Pinus tabulaeformis: CN201410573714.1[P]. Chinese Patent, 2016.
[9] Ramos L Y S, Carballo L M, Melara M V. Establishment of cell suspension cultures of two Costa Rican Jatropha species (Euphorbiaceae)[J]. Revista De Biologia Tropical, 2013, 61(3): 1095−1107.
[10] Merchuk J C. Why use air-lift bioreactors?[J]. Trends Biotechnol, 1990, 8: 66−71. doi: 10.1016/0167-7799(90)90138-N
[11] Kong L S, Holtz C T, Nairn C J, et al. Application of airlift bioreactors to accelerate genetic transformation in American chestnut[J]. Plant Cell Tiss Organ Cult, 2014, 117(1): 39−50. doi: 10.1007/s11240-013-0418-8
[12] 刘春朝, 王玉春, 欧阳藩. 植物组织培养生产有用次生代谢产物的研究进展[J]. 生物技术通报, 1997(5):1−7. Liu C C, Wang Y C, Ouyang P. Advances in the production of useful secondary metabolites by plant tissue culture[J]. Biotechnology Bulletin, 1997(5): 1−7.
[13] 李玲莉, 刘华敏, 孔立生, 等. 利用生物反应器进行香水百合培养研究[J]. 西北林学院学报, 2014, 29(1):89−94. doi: 10.3969/j.issn.1001-7461.2014.01.18 Li L L, Liu H M, Kong L S, et al. Culture of perfume lily in vitro with airlift bio-reactors[J]. Journal of Northwest Forestry University, 2014, 29(1): 89−94. doi: 10.3969/j.issn.1001-7461.2014.01.18
[14] 付双彬. 油松胚性愈伤组织扩增、保存与遗传转化研究[D]. 北京: 北京林业大学, 2016. Fu S B. Proliferation, cryopreservation and genetic transformation of embryogenic callus of Chinese pine (Pinus tabulaeformis C.)[D]. Beijing: Beijing Forestry University, 2016.
[15] Litvay J D, Verma D C, Johnson M A, et al. Influence of a loblolly pine (Pinus taeda L.) culture medium and its components on growth and somatic embryogenesis of the wild carrot (Daucus carota L.)[J]. Plant Cell Report, 1985, 4(6): 325−328. doi: 10.1007/BF00269890
[16] 刘华, 梅兴国. TTC法测定红豆杉细胞活力[J]. 植物生理学通讯, 2001, 37(6):537−539. Liu H, Mei X G. Examining cell viability of Taxus chinensis with TTC (2,3,5-triphenyl-2H-tet-razolium chloride)[J]. Plant Physiology Communications, 2001, 37(6): 537−539.
[17] Jiménez J A, Alonso-Blázquez N, López-Vela D, et al. Influence of culture vessel characteristics and agitation rate on gaseous exchange, hydrodynamic stress, and growth of embryogenic cork oak (Quercus suber L.) cultures[J]. In Vitro Cell & Developmental Biology-Plant, 2011, 47: 578−588.
[18] 吴春霞. 植物细胞悬浮培养的影响因素[J]. 安徽农业科学, 2009, 37(1):36−38. doi: 10.3969/j.issn.0517-6611.2009.01.015 Wu C X. Influencing factors on the culturing of plant suspension Cell[J]. Journal of Anhui Agricultural Sciences, 2009, 37(1): 36−38. doi: 10.3969/j.issn.0517-6611.2009.01.015
[19] 方文娟, 韩烈保, 曾会明. 植物细胞悬浮培养影响因子研究[J]. 生物技术通报, 2005(5):12−15. Fang W J, Han L B, Zeng H M. Research advances in factors affecting establishment of plant cell suspension culture[J]. Biotechnology Bulletin, 2005(5): 12−15.
[20] Somers D A, Birnberg P R, Petersen W L, et al. The effect of conditioned medium on colony formation from ‘black mexican sweet’ corn protoplasts[J]. Plant Science, 1987, 53(3): 249−256. doi: 10.1016/0168-9452(87)90162-2
[21] 孙敬三,桂耀林. 植物细胞工程实验技术[M]. 北京:科学出版社,1995. Sun J S, Gui Y L. Experimental technology of plant cell engineering[M]. Beijing: Science Press, 1995.
[22] Li H, Piao X C, Gao R, et al. Effect of several physicochemical factors on callus biomass and bioactive compound accumulation of R. sachalinensis bioreactor culture[J]. In Vitro Cell & Developmental Biology-Plant, 2016, 52(3): 241−250.
[23] Li Y, Shao C H, Park S Y, et al. Production of salidroside and polysaccharides in Rhodiola sachalinensis using air-lift bioreactor systems[J]. Acta Physiologiae Plantarum, 2014, 36: 2975−2983. doi: 10.1007/s11738-014-1669-7
[24] Jay V, Genestier S, Courduroux J C. Bioreactor studies on the effect of dissolved oxygen concentrations on growth and differentiation of carrot (Daucus carota L.) cell cultures[J]. Plant Cell Report, 1992, 11(12): 605−608.
[25] Tapia E, Sequeida A, Castro A, et al. Development of grapevine somatic embryogenesis using an airlift bioreactor as an efficient tool in the generation of transgenic plants[J]. Journal of Biotechnology, 2009, 139(1): 95−101. doi: 10.1016/j.jbiotec.2008.09.009
[26] Kong L, Tull R, Holtz C T, et al. Application of airlift bioreactors to accelerate genetic transformation of American chestnut[J]. Plant Cell Tissue and Organ Culture, 2014, 117(1): 39−50.
[27] 成喜雨, 何姗姗, 倪文, 等. 植物组织培养生物反应器技术研究进展[J]. 生物加工过程, 2003, 1(2):18−22. doi: 10.3969/j.issn.1672-3678.2003.02.004 Cheng X Y, He S S, Ni W, et al. Advances in bioreactor technology for plant tissue culture[J]. Chinese Journal of Bioprocess Engineering, 2003, 1(2): 18−22. doi: 10.3969/j.issn.1672-3678.2003.02.004



 下载:
下载: