Effects of climate and phylogeny on the relationship between specific leaf area and leaf element concentration of trees and shrubs in Changbai Mountain of northeastern China
-
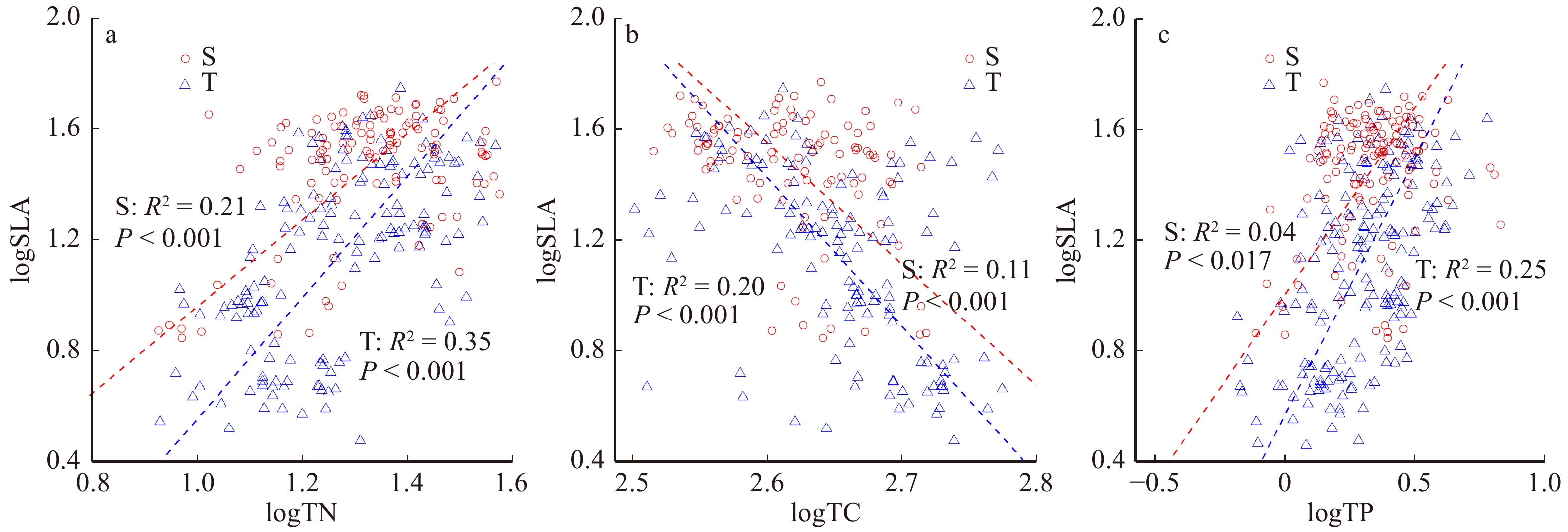
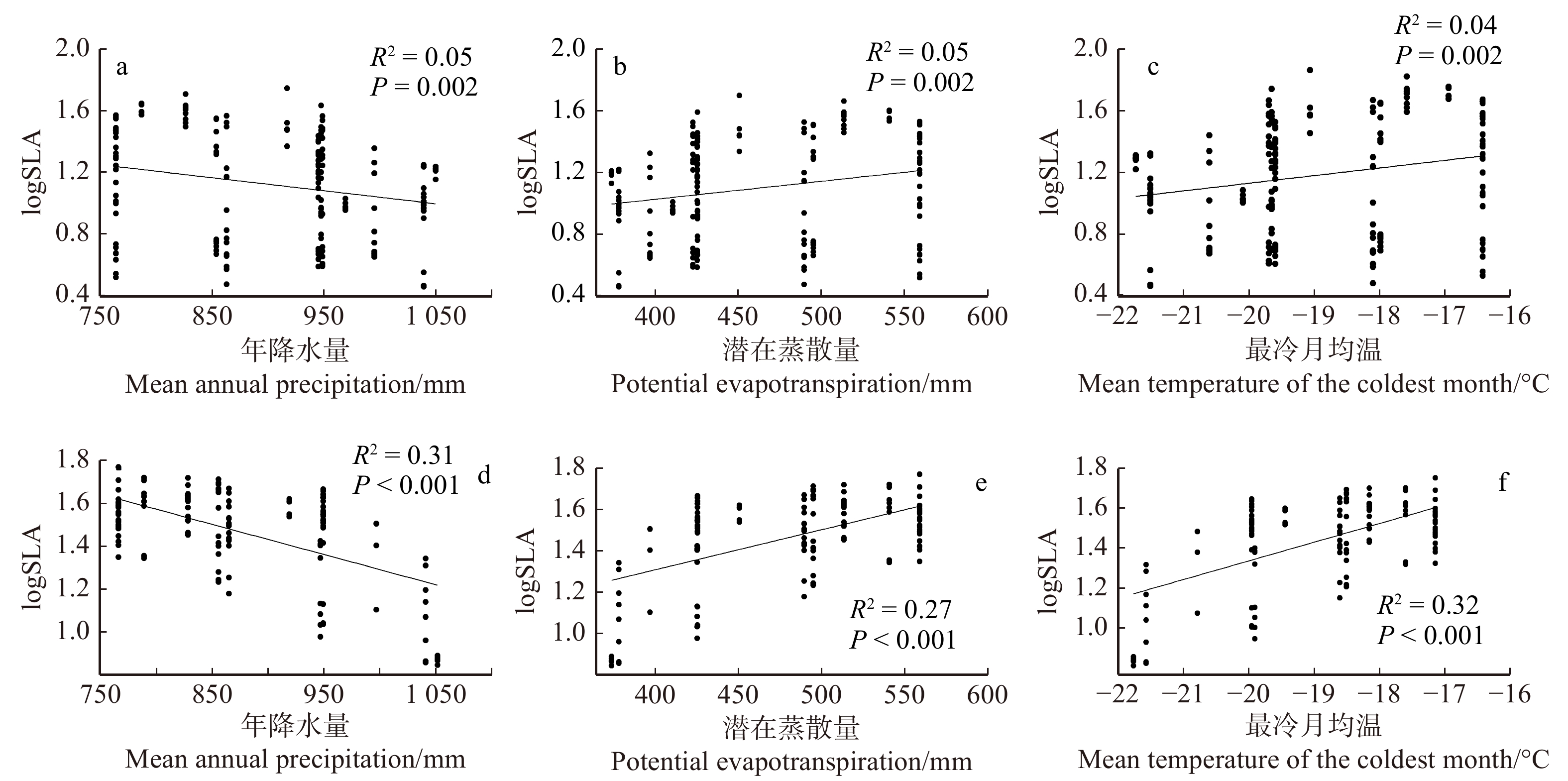
摘要:目的比叶面积(SLA)是植物关键的功能性状,受气候及系统发育影响且与叶片元素含量关系密切,探讨SLA和这些因子的关系,能够反映植物环境适应及资源收支的策略。同时,乔木、灌木是两种不同生活型植物,研究相关的适应策略是否存在差异。方法对长白山海拔梯度上森林样地内常见乔木和林下灌木进行比较,利用DNA条形码序列建立了系统发育树。分析了SLA与叶片元素含量、气候因子和系统发育间的关系,并研究了SLA与叶片元素含量关系及这类关系所受气候和系统发育的影响在乔、灌木间的差异。结果(1) 乔木、灌木SLA均与年降水负相关,与温度条件(最冷月均温、潜在蒸散量)正相关。和灌木相比,乔木SLA受系统发育的影响更大。(2) 乔木、灌木SLA均与TN(全氮)、TP(全磷)含量正相关,与TC(全碳)含量负相关;乔木、灌木SLA和TN、TP间关系的斜率差异显著,和TC间关系的斜率差异不显著。(3) 系统发育影响乔木、灌木SLA与TN、TC间关系的斜率;气候因子影响乔木、灌木SLA与TC间关系的斜率,这两类因子也影响灌木SLA和TP关系的斜率;但乔木SLA和TP关系的斜率不受上述因子影响。结论乔木、灌木SLA对环境的适应及资源收支的策略基本相似,但SLA和TP间关系的影响因子在乔木和灌木中存在一定差异。相对于乔木,林下灌木有趋同进化趋势、SLA普遍较高且对气候更敏感,可能会在叶片内存储更多的磷来满足生存的需要。Abstract:ObjectiveSpecific leaf area (SLA) is a key functional trait of plants. It is affected by climate and phylogeny, and is closely associated with leaf element concentration. Therefore, the relationships between SLA and the abiotic and biotic factors are useful for understanding the strategies of plants in adaptation to environment and resource utilization. Meanwhile, trees and shrubs belong to different life forms, so we need to examine whether they differ in adaptation strategies related to SLA.MethodThis study was based on the comparison of dominant tree and understory shrub species in sample plots along the altitudinal gradient of Changbai Mountain, northeastern China. We established a phylogenetic tree using DNA barcode sequences, and analyzed the relationships of SLA with leaf element concentrations, climatic factors and phylogeny. We examined whether the relationships between SLA and leaf element concentrations, and the influence of climate and phylogeny, were different between trees and shrubs.Result(1) The SLA of trees and shrubs was negatively correlated with mean annual precipitation and positively correlated with thermal conditions (mean temperature of the coldest month and potential evapotranspiration). However, different from shrubs, the SLA of trees was more affected by phylogeny. (2) For both trees and shrubs, SLA was positively correlated with leaf total nitrogen (TN) and total phosphorus (TP) concentrations, but negatively correlated with total carbon (TC) concentration. Trees and shrubs differed significantly in the regression slopes of SLA with TN and TP, but did not differ in the slope of SLA with TC. (3) Climatic factors significantly affected the slope of the relationship between SLA and TC, while phylogeny affected the slope of SLA with TN and TC for both trees and shrubs. Climatic factors and phylogeny together affected the slope between SLA and TP for shrubs, but not for trees.ConclusionThe adaptation of SLA to environment and the resource utilization strategy were similar for trees and shrubs, but there were also differences in the modulators of the relationship between SLA and TP for trees and shrubs. Compared with trees, the understory shrubs have a tendency to convergent evolution, generally with high-SLA leaves and more sensitive to climate, and may store more phosphorus in the leaves to meet the needs of survival.
-
Keywords:
- specific leaf area /
- plant life form /
- leaf element concentration /
- climate /
- DNA barcode /
- plant strategy
-
树木种内不同地理起源的种群间在生长、适应性及形态特征上存在普遍的地理变异(geographic variation),不当的跨生态区调拨种子可导致林分稳定性与生产力降低。造林地区通过对不同原产地或种源的种群做种源试验(provenance trail),阐明种群间的性状变异特点与规律,为造林地区的人工林高效培育选择生长与适应性均佳的优良种源[1]。世界范围的林木种源试验始于20世纪初期[2-3],中国主要造林树种的种源试验多始于80年代,50年代到80年代大规模营造人工林未考虑地理种源因素,树种全分布区内的商业性调种造林现象十分普遍,对跨种子区(seed zone)营建的人工林苗期和幼树的研究显示该现象对林分的稳定性和质量产生不同程度的影响[4]。树木种群是经过数百万年复杂生境的进化产物,具备对环境变化的一定适应性调节能力,且树木种群中也存在较为丰富的个体间适应性遗传变异。引种地生境的选择效应会对外来种群的遗传结构产生影响,由适合个体组成的林分会有更好的稳定性。对不同树龄阶段侧柏(Platycladus orientalis)地理种群的研究显示,早晚期种群的适应性与生长表现了不同规律,且地理种群间的差异随树龄增加而减小,可能是适应性进化与驯化的效应[5]。
油松(Pinus tabuliformis)是有较高经济与生态价值的针叶树种,分布于中国东北、华北、中原和西部地区[6-7],曾是北京周边山区的重要乡土树种[8]。经数千年采伐利用和战乱破坏,至1949年北京及相邻河北北部大范围山地已为荒山或次生林,仅在人际罕至的山顶部有小面积的天然油松林,或皇家园林的百年古树(有可能是北京种源)。20世纪50年代后北京地区启动人工造林项目,至今利用外来种源营建的油松人工林面积已占北京人工林总面积的23.2%[4, 9]。根据有关资料记载1963年—1975年山西省支援北京市油松种子4 × 105 kg,其中关帝和太岳林区为主要供种地[10]。山西省是油松中心分布区,该省内油松主要分布在五山系即管涔山、中条山、太岳山、关帝山和太行山。北京位于油松种子区的东北区,而山西省主要位于中部区[4],不经种源试验的跨种子区用种可能对北京地区油松人工林未来的稳定性与生产力带来不确定影响。北京皇家园林的古油松多长势良好,而一些外来种源营建的人工林则表现了较差的树高长势。研究北京地区油松人工林的遗传多样性和遗传结构的特点与变异,对认识其林分的群体遗传基础、科学管理、利用油松种质以及林木种质遗传多样性的研究具有积极的理论和实际意义。
山西省油松的生长和适应性在山系内林分间近似,在山系间则有显著差异[11-12],各山系种群的遗传结构特征可用序列表达标签(EST-SSR)鉴别[13]。利用EST-SSR分子标记解析北京油松种群的遗传结构,并基于本课题组已有山西五山系地理种群的遗传资料,进一步构建北京种群和山西五山系种群的遗传关联,为推测北京人工林可能的地理种源提供参考。目前利用分子标记的人工林产地溯源鲜有报道,林业发达国家造林对种源有严格规范,很少有种源不清问题,中国除造林单位自采种和良种基地供种外,社会育苗和种子调拨很少关注种源。本研究以北京油松人工林和古油松为试验对象,山西五山系油松地理种群的遗传结构为参照,借助油松核基因组EST-SSR分子标记和种群遗传分析方法,拟(1)揭示北京油松人工林和古油松种群遗传多样性和遗传结构特点;(2)阐明北京油松人工林与古油松的遗传差异;(3)构建北京油松种群与山西五山系油松种群的遗传关联,追溯北京油松人工林种群可能的地理种源;(4)探讨影响人工林种群遗传结构变化的主要因素。为北京地区油松人工林的评价、培育和种质种源管理提供相应信息,对其他树种的人工林溯源亦有一定参考价值。
1. 材料与方法
1.1 试验样地与材料
北京地区油松人工林样本采自十三陵林场(SSL)、八达岭林场(BDL)、九龙山林场(JLS)、密云水库林场(MY)、鹫峰林场(JF)、西山林场(XS)、石湖峪林场(SHY)和妙峰山林场(MFS)共8个种群,北京古油松样本采自以香山公园为中心的皇家园林(GS1)、明十三陵(GS2)和戒台寺(GS3)共3个种群,山西五山系油松地理种群样地基本情况来源于张新波论文资料[14]。4—5月采集当年生针叶(随机取样每株40根),北京人工林的样株间距大于50 m,古油松采集树龄200年以上植株[15],标记样本信息并装于塑封袋,− 20 ℃冰箱中保存备用。各试验林种群地理位置、林龄、林分年均高和采集样本数量见表1。
表 1 供试油松种群基本情况Table 1. General information of sampling stands of Pinus tabuliformis项目
Item种群
Population经度
Longitude纬度
Latitude海拔
Altitude/m树龄/a
Stand age/year年均高
Annual average height/cm样本数
Sample plant number北京人
工林
Beijing plantationJF 116°28′ E 39°54′ N 450 69 14.493 36 JLS 115°59′ E 39°54′ N 751 43 24.419 36 MFS 116°01′ E 40°03′ N 180 45 27.333 30 MY 116°49′ E 40°29′ N 75 45 15.778 36 XS 116°05′ E 40°03′ N 145 64 12.969 35 SSL 116°16′ E 40°15′ N 170 30 21.667 30 BDL 115°56′ E 40°22′ N 676 45 17.111 33 SHY 116°32′ E 40°40′ N 600 59 20.339 36 古油松 Ancient P. tabuliformis GS1 116°10′ E 39°59′ N 135 > 100 — 31 GS2 116°14′ E 40°17′ N 180 > 200 — 102 GS3 116°04′ E 39°52′ N 300 > 200 — 18 山西五
山系
Five mountain populations in Shanxi ProvinceGCS 112°01′ E 38°36′ N 1 650 21 24.762 52 GDS 111°42′ E 37°29′ N 1 655 21 40.476 66 THS 113°31′ E 37°39′ N 1 412 21 27.143 32 TYS 112°04′ E 37°37′ N 1 496 21 33.333 20 ZTS 112°01′ E 35°44′ N 1 519 21 32.381 18 注:JF. 鹫峰林场; JLS. 九龙山林场; MFS. 妙峰山林场; MY. 密云水库; XS. 西山林场; SSL. 十三陵林场; BDL. 八达岭林场; SHY. 石湖峪林场; GS1. 以香山公园为中心的皇家园林; GS2. 明十三陵; GS3. 妙峰山; GCS. 管涔山; GDS. 关帝山; THS. 太行山; TYS. 太岳山; ZTS. 中条山. GS1, GS2, GS3树龄过大,暂不考虑胸径与树高的关系。下同。Notes: JF, Jiufeng Forest Farm; JLS, Jiulongshan Forest Farm; MFS, Miaofengshan Forest Farm; MY, Miyun Reservoir; XS, Xishan Forest Farm; SSL, Shisanling Forest Farm; BDL, Badaling Forest Farm; SHY, Shihuyu Forest Farm; GS1, royal garden centered by Xiangshan Park; GS2, Ming Shisanling Tombs; GS3, Miaofengshan Mountain; GCS, Guancenshan Mountain; GDS, Guandishan Mountain; THS, Taihangshan Mountain; TYS, Taiyuanshan Mountain; ZTS, Zhongtiaoshan Mountain. Since GS1, GS2, and GS3 are too old, the relationship between DBH and tree height is not considered. The same below. 1.2 油松基因组DNA的提取与浓度测定
植物基因组DNA提取试剂盒(离心柱型)(TIANGEN公司,北京)提取样本核基因组DNA。1%琼脂糖凝胶电泳和超微分光光度计(NanoDrop2000, Thermo Fisher Scientific, USA)检测样本DNA的完整性、质量和浓度,置于− 20 ℃冰箱冷冻保存待用。
1.3 引物筛选
引物筛选是在本课题组已有的油松核基因组EST-SSR引物的基础上[13, 16],重复筛选得到的7对多态性高、扩增稳定且重复性好的引物(表2),EST-SSR与传统的从核基因组开发的SSR相比具有低成本高转移性的优点。
表 2 油松PCR检测SSR引物Table 2. SSR primers for PCR detection of P. tabuliformis引物名称
Primer name前引物序列
Former primer sequence (5′→3′)后引物序列
Back primer sequence (5′→3′)荧光修饰
Fluorescent modification片段长度
Fragment length/bpJ9 GTTTGCAGTGAAAGCATGAAAG GCACCAATTCCTTCTCAAATTC HEX 244 ~ 253 J10 GTCGACACTCCAGGGTAGATTC ATATCATCAGCTAATTGTGCGG TAMRA 254 ~ 257 J20 CACCTCCGTAGTTTGATGTTCC CGATGTATCGTGTACACAGCCT FAM 150 ~ 170 J29 AGTCCGAATGTCTTCTTTCTGC TATGGAACGAATCAGAGATGACG FAM 182 ~ 200 J42 AACCTGTCATCCAGTTCCTGTT TTGTCAAATTCCAATTCAGCAC TAMRA 251 ~ 269 J48 GAAGAGGAAGACGAAATGGATG CTTTACATTTACCGCCTCTGCT ROX 262 ~ 268 J50 TCATCCATTTCAATAGCACGAC GTAGCTGCTTGGCCTGATTATC HEX 235 ~ 244 注:引自参考文献[16]。Note: cited from reference [16]. 1.4 SSR-PCR反应体系和程序
PCR体系分为两步,第一步反应体系为10 µL,包括样本DNA 1 µL,前、后引物各0.4 µL,优博兰2 × Taq Mixture 5 µL,ddH2O补足10 µL。PCR的扩增程序为:95 ℃预变性5 min;95 ℃变性30 s,65 ℃退火30 s,72 ℃延伸30 s,该过程循环35次;最后,72 ℃延伸10 min,4 ℃保存。第二步反应体系为20 µL,包括第一步产物3 µL,带有荧光染料的M13接头和后引物各0.6 µL,优博兰2 ×Taq Mixture 10 µL,ddH2O补足20 µL。PCR的扩增程序为:95 ℃预变性5 min;95 ℃变性30 s,58 ℃退火30 s,72 ℃延伸30 s,该过程循环33次;最后,72 ℃延伸10 min,4 ℃保存。
1.5 数据分析
PCR扩增产物由北京睿博兴科生物技术有限公司用毛细管电泳法检测,用GeneMarker 2.2.0(https://softgenetics.com/GeneMarker.php)软件进行峰值读取,Excel记录结果数据;通过POPGENE 1. 3. 1(https://www.biocompare.com/)软件,进行种群遗传变异与遗传多样性参数分析,包括观测等位标记数(Na)、有效等位标记数(Ne)、观测杂合度(Ho)、期望杂合度(He)和Shannon’s信息指数(I);对不同种群之间的遗传结构差异进行分析,包括群体内的固定指数(FIS)、总群体的固定指数(FIT)、群体间遗传分化系数(FST)和基因流(Nm),得到不同种群间的遗传一致度(genetic identity, GI)和遗传距离(genetic distance, GD)等参数,运用MEGA 7.0(https://www.megasoftware.net/)软件基于Nei’s遗传距离生成UPGMA聚类图;计算各位点间差异显著性。
2. 结果与分析
2.1 SSR位点在16个种群中扩增的多态性分析
7对引物检测北京和山西16个油松种群658株样本,扩增结果见表3,7个SSR位点在全部样本中共检测出23个等位标记数目,各种群等位标记数目范围为16 ~ 22个,种群JLS, GS3和ZTS的等位标记数最少只有16个,种群GCS的等位标记数最多有22个;除位点J29在种群JF、MFS、XS和GS3中无多态性,其余位点均在不同种群中扩增出2 ~ 4个等位标记数;位点J29在不同种群间的等位标记频率差异很小,各种群间扩增情况相似;等位标记J42-A和J42-D在不同种群中扩增频率差异大,依次波动于0.0% ~ 50.0%和40.3% ~ 94.8%之间;等位标记J10-A在北京人工林、古油松和山西五山系中频率差异很大,在北京人工林种群和古油松种群中约有90%的扩增,而在山西五山系中只有65%的扩增;等位标记J20-D、J50-C和J50-D在北京人工林种群和山西五山系中均有低频扩增,而在北京古油松中的扩增为0;即:同一位点在不同种群中的等位标记多态性存在差异,同样,各位点在同一种群中的等位标记多态性也存在差异。
表 3 16个油松种群各位点等位标记频率Table 3. Allele frequency of each locus in the 16 populations of P. tabuliformis引物 Primer 等位标记 Allele marker 北京人工林
Beijing plantation古油松
Ancient P. tabuliformis山西五山系
Five mountain populations
in Shanxi ProvinceJF JLS MFS MY XS SSL BDL SHY GS1 GS2 GS3 GCS GDS THS TYS ZTS J9 A 0.281 0.375 0.267 0.235 0.242 0.014 0.106 0.043 0.133 0.189 0.222 0.144 0.242 0.219 0.150 0.167 B 0.719 0.625 0.733 0.765 0.758 0.987 0.894 0.957 0.867 0.811 0.778 0.856 0.758 0.781 0.850 0.833 J10 A 0.941 0.985 0.981 0.956 0.982 0.865 0.879 0.917 0.983 0.995 0.972 0.692 0.677 0.609 0.675 0.750 B 0.059 0.015 0.019 0.044 0.018 0.135 0.121 0.083 0.017 0.005 0.028 0.308 0.323 0.391 0.325 0.250 J20 A 0.015 — 0.019 0.106 0.056 0.028 0.016 — 0.035 0.026 — 0.040 0.074 0.048 0.079 0.094 B 0.632 0.786 0.904 0.697 0.852 0.778 0.688 0.889 0.931 0.871 0.941 0.890 0.787 0.774 0.842 0.594 C 0.353 0.214 0.077 0.167 0.093 0.153 0.297 0.111 0.035 0.103 0.059 0.070 0.131 0.129 0.079 0.313 D — — — 0.030 — 0.042 — — — — — — 0.008 0.048 — — J29 A — — — 0.015 — 0.015 0.206 0.063 0.024 0.014 — 0.010 0.015 — — — B 1.000 0.881 1.000 0.970 1.000 0.941 0.677 0.906 0.857 0.892 1.000 0.933 0.879 0.984 0.950 0.944 C — 0.095 — 0.015 — — 0.118 0.031 0.119 0.081 — 0.048 0.099 — 0.050 0.056 D — 0.024 — — — 0.044 — — — 0.014 — 0.010 0.008 0.016 — — J42 A 0.167 0.500 0.017 0.329 — 0.014 0.210 0.014 0.050 0.015 0.028 0.010 — 0.016 — — B 0.046 — 0.017 0.014 0.016 0.069 0.032 0.014 0.050 0.069 0.056 0.039 0.048 0.177 0.025 0.028 C 0.015 — 0.017 0.043 0.047 0.264 0.210 0.306 0.383 0.157 0.250 0.206 0.318 0.403 0.225 0.222 D 0.773 0.500 0.948 0.614 0.938 0.653 0.548 0.667 0.517 0.760 0.667 0.745 0.635 0.403 0.750 0.750 J48 A 0.206 0.208 0.100 0.177 0.132 0.176 0.197 0.194 0.242 0.142 0.222 0.200 0.269 0.313 0.290 0.028 B 0.721 0.736 0.800 0.691 0.765 0.757 0.727 0.722 0.710 0.784 0.722 0.750 0.723 0.688 0.684 0.972 C 0.074 0.056 0.100 0.132 0.103 0.068 0.076 0.083 0.048 0.074 0.056 0.050 0.008 — 0.026 — J50 A 0.221 0.157 0.232 0.177 0.206 0.216 0.242 0.250 0.183 0.314 0.361 0.125 0.156 0.188 0.175 0.167 B 0.779 0.843 0.768 0.824 0.750 0.743 0.758 0.722 0.817 0.686 0.639 0.875 0.828 0.797 0.825 0.833 C — — — — 0.029 — — — — — — — 0.008 0.016 — — D — — — — 0.015 0.041 — 0.028 — — — — 0.008 — — — 合计Total 23 17 16 17 20 18 21 19 19 19 19 16 20 22 19 17 16 注:各等位SSR标记排序按照扩增产物片段从小到大的顺序;山西省五山系油松地理种群的各位点多样性数据来源于武文斌试验原始数据[13]。Notes: each allelic SSR markers are sorted according to the order of amplification product fragments from small to large; the diversity data of the geographical distribution of P. tabuliformis in Shanxi Province are derived from the original data of professor Wu Wenbin[13]. 对16个油松种群等位标记频率差异性进行卡方检验,7个位点等位标记频率差异显著(P < 0.05),试验数据可以用于进行下一步分析。
2.2 油松种群遗传多样性
对16个油松种群的遗传多样性分析见表4,Ne、He和I 3个指标常用来衡量遗传多样性。不同种群的Na和Ne依次是2.286 ~ 3.143和1.297 ~ 1.732,Na种群GDS最高,种群JLS和ZTS最低,Ne种群BDL最高,种群MFS最低;I变化于0.354 ~ 0.661,种群BDL最高,种群MFS最低;Ho和He分别为0.210 ~ 0.428和0.204 ~ 0.397,Ho种群TYS最高,种群XS和SSL最低,He种群BDL最高,种群MFS最低;Hardy-Weinberg平衡偏离指数(D)可由和计算得到D = (Ho − He)/He,D > 0时,表现为杂合子过剩,D < 0时,表现为杂合子缺失;计算各种群的Hardy-Weinberg平衡偏离指数,其中种群XS、SSL、BDL、GS1和GDS的D < 0,表现杂合子缺失,在剩余11个种群中D > 0,表现杂合子过剩;极值比可以反应各种群间遗传结构差异的程度,除在Na中表现为山西五山系种群 > 北京人工林种群 > 古油松,Ne、I、Ho和He均是北京人工林种群 > 山西五山系种群 > 古油松,说明北京油松人工林种群之间遗传差异最大,古油松之间的遗传差异最小。
表 4 16个油松种群遗传多样性Table 4. Genetic diversity of 16 P. tabuliformis populations项目 Item 种群 Population Na Ne I Ho He D 北京人工林
Beijing plantationJF 2.429 1.513 0.503 0.367 0.310 0.184 JLS 2.286 1.536 0.503 0.417 0.323 0.291 MFS 2.429 1.297 0.354 0.237 0.204 0.162 MY 2.857 1.569 0.562 0.343 0.327 0.049 XS 2.571 1.341 0.406 0.210 0.227 − 0.075 SSL 3.000 1.479 0.528 0.210 0.294 − 0.286 BDL 2.714 1.732 0.661 0.271 0.397 − 0.317 SHY 2.857 1.437 0.482 0.414 0.278 0.489 极值比
Extremum ratio1.312 1.335 1.867 1.985 1.946 — 古油松
Ancient P. tabuliformisGS1 2.857 1.487 0.496 0.239 0.285 − 0.161 GS2 2.857 1.423 0.488 0.310 0.277 0.119 GS3 2.286 1.467 0.449 0.303 0.227 0.094 极值比
Extremum ratio1.249 1.044 1.104 1.297 1.028 — 山西五山系
Five mountain populations in Shanxi ProvinceGCS 2.857 1.439 0.504 0.302 0.291 0.038 GDS 3.143 1.608 0.608 0.315 0.370 − 0.149 THS 2.714 1.731 0.617 0.400 0.382 0.047 TYS 2.429 1.494 0.517 0.428 0.321 0.333 ZTS 2.286 1.479 0.479 0.311 0.297 0.047 极值比
Extremum ratio1.374 1.202 1.288 1.417 1.312 — 注:Na. 观测等位标记数;Ne. 有效等位标记数;I. Shannon’s信息指数;Ho. 观测杂合度;He. 期望杂合度;D. Hardy-Weinberg平衡偏离指数;极值比:在同一大类地理种群中某一参数值的最大值与最小值的比值。Notes: Na, number of observed alleles; Ne, effective alleles; I, Shannon’s information index; Ho, observed heterozygosity; He, expected heterozygosity; D, Hardy-Weinberg balance deviation index; extremum ratio: ratio of the maximum value to the minimum value of a parameter value in the same large geographic population. 2.3 各位点在油松种群中遗传分化
7个位点在16个油松种群间的遗传分化结果见表5,除位点J20、J29和J50外,其余各位点FIS均为负值,平均为− 0.076,说明样本油松群体内杂合子过剩,其中古油松FIS为− 0.102,种群内杂合子过剩最多,而山西五山系FIS为− 0.068,种群内杂合子过剩最少;FIT在J9、J10和J48 3个位点中为负值,即FIT < 0,其余4个位点FIT > 0,平均为− 0.001,表明油松总样本种群杂合子过剩,其中古油松FIT是− 0.077,北京人工林FIT是− 0.001;FST在各位点间波动于0.021 ~ 0.151,平均为0.070,即平均遗传分化为7%,表明7%的遗传变异存在种群间,而93%的遗传变异存在种群内,种群内的变异是遗传变异的主要来源,其中人工林FST高达0.066,种群间存在中等程度的遗传结构变异,而古油松和山西五山系种群间遗传结构变异程度较低,FST依次是0.023和0.033[17],北京人工林种群之间遗传结构差异最大,古油松种群间遗传结构差异最小,该结果和极值比的结果一致。
表 5 16个油松种群中的F统计量Table 5. F-statistics in 16 P. tabuliformis populations参数
ParameterJ9 J10 J20 J29 J42 J48 J50 平均值 Mean 北京人工林 Beijing plantation 古油松
Ancient P. tabuliformis山西五山系
Five mountain populations
in Shanxi ProvinceFIS − 0.282 − 0.360 0.217 0.228 − 0.057 − 0.193 0.016 − 0.076 − 0.071 − 0.102 − 0.068 FIT − 0.217 − 0.155 0.266 0.287 0.068 − 0.162 0.005 − 0.001 − 0.001 − 0.077 − 0.033 FST 0.051 0.151 0.062 0.078 0.119 0.026 0.021 0.070 0.066 0.023 0.033 Nm 4.657 1.402 3.763 2.973 1.860 9.328 11.593 3.314 3.552 10.806 7.311 注:FIS. 群体内的固定指数;FIT. 总群体的固定指数;FST. 群体间遗传分化系数;Nm. 基因流。Notes: FIS, fixed index in population; FIT, fixed index of total population; FST, coefficient of genetic differentiation among populations ; Nm, gene flow. 2.4 种群间遗传距离与系统关系
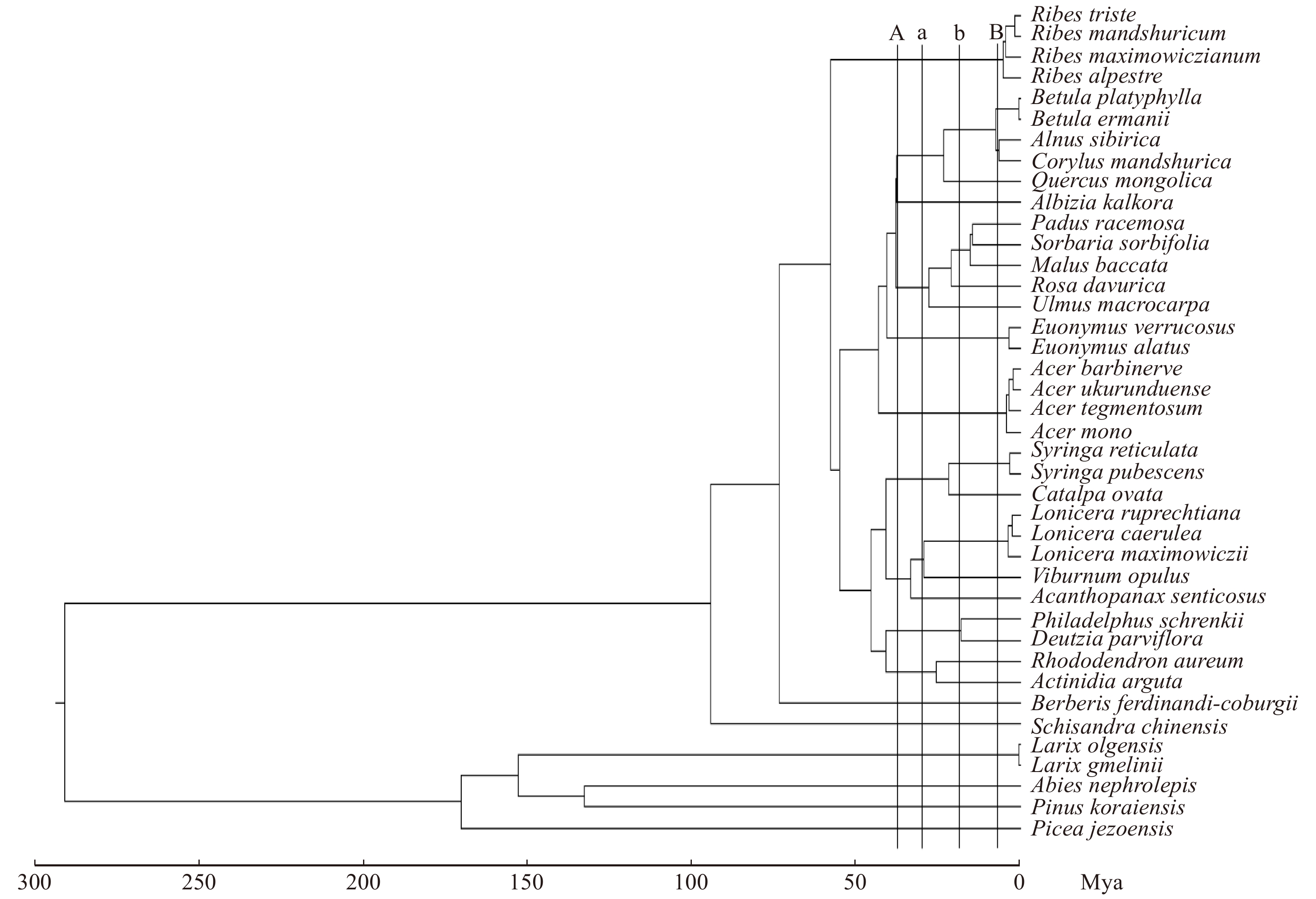
遗传距离(GD)可衡量种群间亲缘关系远近,遗传一致度(GI)是衡量种群间遗传相似性参数,表6显示16个油松种群间遗传距离和遗传一致度变化范围为0.009 ~ 0.093和0.911 ~ 0.999,说明种群间遗传相似程度较高,遗传距离较小。基于样本16个油松种群间的Nei’s遗传距离通过软件MEGA7.0进行UPGMA聚类分析,结果见图1,可以看出当遗传距离在0.020左右时,16个油松种群可以分为3大类。第一大类(Ⅰ类)包括10个种群,其中古油松GS1、GS2和GS3与北京人工林SSL和SHY在遗传距离0.010处聚为第一亚类,而后与北京人工林JF、MY、MFS和XS一起聚为第一大类;第二大类(Ⅱ类)由山西的五山系种群构成,并显示山系种群间有一定遗传距离,其中位于山西省中部的3个种群GDS、GCS和TYS间具有较近遗传距离;北京人工林种群JLS单独构成第三大类(Ⅲ类)。
表 6 北京11个油松种群和山西五山系油松种群的遗传一致度和Nei’s遗传距离Table 6. Genetic identity and genetic distance of 11 P. tabuliformis populations in Beijing and P. tabuliformis populations in five mountain populations in Shanxi Province种群 Population JF JLS MFS MY XS SSL BDL SHY GS1 GS2 GS3 GCS GDS THS TYS ZTS JF — 0.971 0.981 0.988 0.983 0.967 0.966 0.962 0.950 0.977 0.969 0.960 0.958 0.931 0.960 0.970 JLS 0.030 — 0.951 0.986 0.949 0.932 0.956 0.935 0.946 0.952 0.948 0.932 0.934 0.911 0.927 0.927 MFS 0.019 0.051 — 0.975 0.999 0.969 0.950 0.973 0.961 0.991 0.983 0.971 0.959 0.922 0.967 0.962 MY 0.012 0.014 0.026 — 0.976 0.969 0.972 0.967 0.966 0.976 0.970 0.962 0.958 0.936 0.959 0.958 XS 0.017 0.053 0.001 0.024 — 0.974 0.953 0.976 0.964 0.992 0.983 0.973 0.962 0.926 0.970 0.964 SSL 0.034 0.070 0.032 0.031 0.027 — 0.978 0.996 0.983 0.984 0.981 0.984 0.978 0.965 0.983 0.976 BDL 0.034 0.046 0.051 0.028 0.048 0.022 — 0.979 0.973 0.972 0.961 0.962 0.963 0.942 0.959 0.964 SHY 0.039 0.067 0.028 0.033 0.024 0.004 0.021 — 0.991 0.989 0.989 0.982 0.976 0.959 0.980 0.964 GS1 0.059 0.055 0.040 0.035 0.037 0.017 0.028 0.009 — 0.982 0.985 0.972 0.974 0.960 0.970 0.948 GS2 0.023 0.049 0.009 0.025 0.008 0.016 0.029 0.011 0.018 — 0.994 0.973 0.968 0.939 0.970 0.965 GS3 0.032 0.053 0.018 0.030 0.017 0.019 0.040 0.012 0.016 0.006 — 0.970 0.969 0.953 0.971 0.951 GCS 0.041 0.070 0.029 0.039 0.027 0.016 0.039 0.019 0.028 0.027 0.030 — 0.994 0.975 0.998 0.976 GDS 0.043 0.069 0.041 0.043 0.039 0.022 0.038 0.025 0.027 0.033 0.032 0.006 — 0.987 0.995 0.976 THS 0.072 0.093 0.081 0.066 0.077 0.036 0.060 0.042 0.041 0.063 0.049 0.025 0.013 — 0.978 0.953 TYS 0.041 0.076 0.033 0.042 0.030 0.018 0.042 0.020 0.031 0.030 0.030 0.002 0.005 0.022 — 0.972 ZTS 0.030 0.076 0.039 0.043 0.036 0.025 0.037 0.036 0.053 0.036 0.050 0.025 0.024 0.048 0.029 — 注:对角线以上为遗传相似度,对角线以下为遗传距离。Notes: above diagonal represents Nei’s genetic identity, below diagonal represents genetic distance. 3. 讨 论
3.1 北京油松人工林的遗传结构及其与古油松种群差异
8个北京油松人工林种群在遗传结构上均表现出偏离遗传平衡的特点。种群JF、JLS、MFS、MY和SHY表现为杂合子过剩,种群XS、SSL和BDL则表现为杂合子不足的状态,说明引种地环境的选择效应与人工林培育过程的驯化作用对群体的遗传结构产生了不同的影响。样本中所有种群的期望杂合度远大于Vendramin等[18]所提出的遗传衰退临界值(H < 0.05),人工林各种群期望杂合度普遍大于古油松各种群期望杂合度有更高的遗传多样性,说明引入的外来油松种群在幼龄至成林阶段经受了较大的选择压力,而位点杂合度较高个体有更大的选择适合度。北京古油松主要分布于皇家园林、帝王陵墓或者寺庙等地,虽然为人工林,但其种源没有记载,由于百年以前北京周边山区仍有当地起源油松林,择优采种育苗,用于皇家园林营建是有可能的。且限于当时的交通条件,经由他省为古油松造林调种的可能性较小,故皇家园林的古油松可能源于北京本地。古油松种群的遗传结构亦均处于非遗传平衡状态,并表现出较高的杂合子过剩,反映了北京皇家园林的古油松种群经过数百年栽培驯化后的种群遗传结构特性,样本植株有较高的杂合度,推测利杂的适合度可能是较高杂合度主因,且景观需要也可能是其保留的人为因素。从一个侧面也反映了在油松人工林的生长过程中选择与驯化作用具有一定的利杂趋势[19-20]。
7对引物的分析结果表明北京油松人工林种群遗传多样性普遍大于古油松种群遗传多样性,也显示古油松有效种群的规模有限,遗传型有共同起源与选择效应的特点。北京油松人工林种群的有效等位标记数、期望杂合度和Shannon’s多样性指数3个指标在3类种群中均为最高值,故遗传多样性最高,这与人工林种质可能来自不同山系种源、人工林的抚育方法、不同种群间生境的差异以及不同来源种群的驯化效应不同有关。虽然油松人工林的遗传变异主要存在于种群内,符合大多数林木种群遗传变异的遗传结构特征和规律[21-22],但与山西五山系种群和古油松种群相比,有更高的种群间遗传变异,也从另一侧面说明了油松人工林种群的种质来源与进化因素影响的复杂性。
3.2 北京油松人工林和山西五山系油松种群的遗传结构差异及其遗传关联
同一位点在不同种群中等位标记频率差异是种群间遗传多样性和遗传结构变异的基础[23-24]。由表3不同位点在样本种群中的检测结果可知,各等位标记频率在不同类别油松种群中呈现出一定的差异趋势,等位标记J10-A在北京地区油松人工林和古油松种群中的扩增频率相似且很高,而在山西五山系油松种群中扩增频率较低;J42-A在北京人工林中频率较高,在古油松群体中频率则较低,而在山西五山系种群中有最低值且部分群体为零。位点J10、J20、J42和J50较位点J9、J29和J48在3类种群中的等位标记检测频率差别较大,可能对生境选择更敏感。同一位点不同等位标记在不同种群间、种群类别间检测到的频率差异是检测种群间遗传结构差异和遗传分化的基础,而非中性标记(如部分EST-SSR标记)检测结果在一定程度上可以反映生境对种群的选择效应。等位标记J20-B在北京人工林种群和山西五山系种群的扩增频率相似,与古油松存在明显差异,说明该等位标记为北京人工林和山西种群的共同特征,由于两者具有共同来源关系,该标记的稳定表现,可能没有受北京生境选择影响。
3类油松种群间的遗传距离表现了亲缘关系的相对远近。16个油松种群被分为3大类,其中古油松GS1、GS2和GS3位于第一大类(Ⅰ类)的第一亚类内,其他北京人工林(除JLS)同聚在第一大类内,而山西的五山系种群则均处于第二大类(Ⅱ类)内,显示北京地区的油松人工林和古油松间具有相对较近的亲缘关系,并与山西五山系油松种群间有一定亲缘差异,根据我们的调查资料显示[10],北京地区近代的油松人工林种子多来自山西种源,特别是当年主要供种的关帝山(GDS)和太岳山(TYS),从人工林的种质溯源分析角度看,两者间应该有较密切的亲缘关系,而我们的分析结果却不能提供可靠的溯源证据,这可能与我们采用的分子标记(EST-SSR)有关。
北京油松人工林种群未能按照试验预期与山西五山系种源溯源,导致这一结果的可能原因有:山西五山系的油松生长在夏季凉爽湿润的1 300 ~ 1 400 m高海拔气候环境,而北京油松引种地多是100 ~ 200 m低山,夏季高温湿热,较山西地区温度高且降水多。自然气候条件的差异对不同个体产生选择压力,种群内不同个体间的适合度存在差异,油松种子适应度差异表现在从种子发芽[25]、生长、发育到形成成熟植株,各个阶段能够存活下来的植株适应北京地区生境气候条件,不适应的则死亡或者被淘汰,试验选择的对象树龄多在40 ~ 60年,已经经过苗期到成林阶段的北京地区低海拔生境强度选择保留下来的种群,由于这个种群的许多个体遭到淘汰,种群的遗传结构势必要遭到改变[26]。本试验借助的EST-SSR分子标记是来源于核基因组编码区的非中性共显性标记,由于引入种群在较大差异生境下不同遗传型的适合度差异可导致其遗传结构改变,而所用编码区标记中一些可能与适合度关联,故表现了北京人工林种群有别与供种地种群的遗传结构特点。利用这些标记对山西各山系地理种群遗传结构解析中发现,种群遗传结构的变异与生境的湿热比密切相关[13],间接说明这些标记与生境存在一定关联,但因缺乏油松功能基因组信息,现尚不能找到这些EST-SSR标记所关联的基因功能。因此,对已有基因组信息物种的相关研究,在利用EST-SSR标记解析种群遗传结构和构建种群间系统学关系时,应尽量了解这些编码区标记所关联基因的功能,利用与适合度关联标记研究现实种群遗传结构与生态效应,利用与非适合度关联标记构建种群间系统关系。北京九龙山种群(JLS)在北京人工林种群中海拔位置最高(750 m),相对较高海拔高度与偏北的纬度效应,可能使引种地对外来种质的进化影响变小,故而与山西五山系种群间相对北京种群有更近遗传距离。
树高是评价森林生产力的重要指标[27],北京油松人工林年均高生长量在12.969 ~ 27.333 cm,山西五山系油松林年均高生长量在24.763 ~ 40.476 cm,显示为北京油松人工林树高生长显著低于山西油松生长水平,说明山西高海拔生境更适宜于油松林分生长,而北京的低山生境则对油松的生长量有显著的胁迫影响,两地海拔导致的气象因子显著差异可能是影响油松生产力的主因,而这种高、低海拔生境的差异势必对种群中个体的适合度产生显著影响,进而导致种群遗传结构的改变,使引入的人工林种群表现了普遍偏离遗传平衡状态的现象。因此,也从种群遗传结构变化角度,说明海拔高度对于油松的适应性与生长表现有较大的影响。
3.3 利用编码序列EST-SSR标记进行人工林亲缘关系鉴定的可能性与局限
北京人工林种群与古油松在遗传距离上更近,离山西五山系的遗传关系较远,山西五山系种质在北京引种地胁迫生境下的适合度差异导致部分植株被淘汰,因此保留下来的种群遗传结构发生一定程度的改变。而遗传结构改变的趋势将会有利于山西五山系种群逐步适应引入地生境,形成遗传结构区别于山西五山系和北京古油松的种群。本研究通过油松核基因组7对EST-SSR标记虽未能准确推断出与文献记载相一致的北京油松人工林种源,但可以确定不同种群之间的相对亲缘关系[28]。外来种质在引入地胁迫生境下的适合度差异导致部分植株被淘汰,因此保留下来的种群的遗传结构发生一定程度的改变,所以,用核基因组基因来进行种群溯源会存在一定程度的偏差,导致溯源结果不理想。种子的传播会对遗传的空间结构产生影响[29],而细胞质基因组突变率低、在上下代间传递很稳定,且油松线粒体DNA(mtDNA)属于母本遗传,mtDNA标记的空间结构完全由种子传播决定。所以,试验下一阶段将通过线粒体DNA的序列[30-31]构建人工林油松和山西五山系的亲缘关系,在已有核基因组SSR基础之上结合mtDNA遗传标记来研究北京油松种群的遗传多样性、遗传结构和溯源问题。同时扩大试验亲本种源区范围,为北京市的油松人工林最佳种源的选择提供理论基础。
本研究发现北京油松人工林种群较古油松种群变异更丰富,遗传多样性更高。北京油松人工林和古油松种群的分析表明遗传变异主要存在于种群内,种群间的变异低于种群内遗传变异。研究群体普遍存在偏离遗传平衡现象,位点J10、J20、J42和J50在北京人工林、古油松和山西五山系中位点扩增频率差异显著,可作为北京人工林种源溯源的关键引物。引种地对种群的选择效应可导致种群遗传结构改变,利用基因编码区的EST-SSR标记做种群溯源有局限性。研究结果为北京地区油松人工林的评价、培育和种质种源管理提供相应信息,对其他树种的人工林溯源亦有一定参考价值。
-
表 1 长白山样地信息表
Table 1 General information of the sample plots on Changbai Mountain
样地编号
Sample plot No.经度
Longitude (E)纬度
Latitude (N)海拔
Altitude/m林型
Forest typeCB01 128°11′ 42°39′ 530 白桦林 Betula platyphylla forest CB02 128°08′ 42°41′ 650 阔叶红松林 Mixed broadleaved-Korean pine forest CB03 128°12′ 42°32′ 840 阔叶红松林 Mixed broadleaved-Korean pine forest CB04 128°17′ 42°23′ 950 白桦林 Betula platyphylla forest CB05 128°17′ 42°23′ 970 阔叶红松林 Mixed broadleaved-Korean pine forest CB06 128°17′ 42°19′ 1 010 云冷杉林 Picea-Abies forest CB07 128°11′ 42°12′ 1 270 阔叶红松林 Mixed broadleaved-Korean pine forest CB08 128°26′ 42°07′ 1 420 云冷杉林 Picea-Abies forest CB09 128°09′ 42°10′ 1 420 长白落叶松林 Larix olgensis forest CB10 128°24′ 42°08′ 1 440 长白落叶松林 Larix olgensis forest CB11 128°08′ 42°09′ 1 530 长白落叶松林 Larix olgensis forest CB12 128°07′ 42°07′ 1 660 长白落叶松林 Larix olgensis forest CB13 128°07′ 42°06′ 1 885 岳桦林 Betula ermanii forest CB14 128°07′ 42°06′ 1 940 岳桦林 Betula ermanii forest 表 2 GeneBank登录号
Table 2 GeneBank accession No.
种
Species替代项
Species substitute序列号 GeneBank accession No. rbcL matK ITS Abies nephrolepis AB029643.1 JQ512390.1 EF057712.1 Acer ukurunduense Acer caudatum (ITS) DQ978402.1 AB872532.1 AY605435.1 Acer tegmentosum DQ978437.1 KU902540.1 KU902470.1 Acer mono DQ978416.1 KX264941.1 KX494362.1 Acer barbinerve Acer stachyophyllum (matK) DQ978395.1 MH116502.1 KY649432.1 Albizia kalkora HQ427141.1 HQ427295.1 JF708202.1 Alnus sibirica FJ844576.1 AB015456.1 AY352323.1 Acanthopanax senticosus GQ436704.1 KU378100.1 AF077885.1 Actinidia arguta KR819563.1 AF322596.1 KP314034.1 Betula ermanii KF418937.1 AY372016.1 AY761108.1 Betula platyphylla AY263927.1 AY372023.1 AY761128.1 Berberis ferdinandi-coburgii KC788474.1 JQ172855.1 KC575607.1 Catalpa ovata KP088517.1 KX526591.1 AY486304.1 Corylus mandshurica KF418954.1 KF419041.1 FJ011749.1 Deutzia parviflora KP120368.1 KP120240.1 KX074137.1 Euonymus verrucosus Euonymus nitidus (rbcL) KT258953.1 HQ393842.1 KF282206.1 Euonymus alatus L13184.2 EF135537.1 KF282156.1 Juniperus squamata HM024339.1 HM024061.1 GQ118644.1 Larix gmelinii AB303666.1 JQ512433.1 AY523449.1 Larix olgensis JQ512557.1 JQ512433.1 AY523449.1 Lonicera caerulea Lonicera microphylla (rbcL) KP088666.1 KU673053.1 KU954293.1 Lonicera maximowiczii Lonicera lanceolata (rbcL) MH116245.1 GU168652.1 FJ217878.1 Lonicera ruprechtiana Lonicera maackii (rbcL) KP088665.1 FJ745394.1 FJ217828.1 Malus baccata JQ391376.1 MG220601.1 JQ392457.1 Padus racemosa AF411485.1 GU363752.1 KX013510.1 Pinus koraiensis JQ512580.1 AB161009.1 KC583357.1 Picea jezoensis JQ512568.1 JQ512443.1 AF024627.2 Philadelphus schrenkii KP120309.1 KF202024.1 KP120051.1 Rhododendron aureum Rhododendron williamsianum (rbcL) KM606388.1 AY494177.1 AF393409.1 Rosa davurica Rosa bella (rbcL) GQ436585.1 JN566087.1 KP994574.1 Ribes triste MG249508.1 JN966506.1 AF426319.1 Ribes mandshuricum Ribes rubrum (rbcL) KM360958.1 KX676698.1 AF426320.1 Ribes maximowiczianum JN102268.1 JN102200.1 AF426376.1 Ribes alpestre JF944124.1 JF956163.1 JF978471.1 Quercus mongolica AB060584.1 AB107631.1 KX838276.1 Sorbaria sorbifolia MG703612.1 AF288125.1 JQ041771.1 Syringa pubescens Syringa oblata (rbcL) KP088868.1 JN590997.1 DQ022416.1 Syringa reticulata KP088871.1 MG772989.1 DQ022417.1 Schisandra chinensis AF238061.1 JF956213.1 KX815927.1 Ulmus macrocarpa Ulmus pumila (ITS) JF317495.1 JF317435.1 KC539582.1 Viburnum opulus HQ591762.1 MG225093.1 JQ805158.1 表 3 叶片性状数据描述
Table 3 Description of leaf trait data
叶性状
Leaf trait生活型
Life form平均值
Mean标准偏差
SD变异系数
CV比叶面积 乔木 Tree 17.42b 11.92 0.68 SLA/(m2·kg− 1) 灌木 Shrub 31.72a 11.96 0.38 全氮含量 乔木 Tree 20.5 6.92 0.34 TN/(g·kg− 1) 灌木 Shrub 21.82 6.67 0.31 全碳含量 乔木 Tree 454.83a 61.45 0.14 TC/(g·kg− 1) 灌木 Shrub 413.45b 47.2 0.11 全磷含量 乔木 Tree 2.18 0.88 0.4 TP/(g·kg− 1) 灌木 Shrub 2.21 0.89 0.41 氮磷比 乔木 Tree 10.44 4.8 0.46 N:P 灌木 Shrub 11.04 4.7 0.43 注:不同字母表示不同生活型植物性状测量值有显著差异(P < 0.05)。下同。Notes: different letters indicate significant differences in measured trait values of different life forms (P < 0.05). SLA, specific leaf area; TN, total nitrogen concentration; TC, total carbon concentration; TP, total phosphorus concentration. The same below. 表 4 SMA斜率差异性检验
Table 4 Testing the difference of SMA slope
相关关系
Correlation乔木
Tree灌木
ShrubP logSLA − logTN 2.19 (1.92 ~ 2.50) 1.56 (1.33 ~ 1.85) 0.002 logSLA − logTC − 5.37 (− 4.59 ~ − 6.28) − 4.37 (− 3.63 ~ − 5.28) 0.1 logSLA − logTP 1.85 (1.63 ~ 2.11) 1.35 (1.13 ~ 1.60) 0.004 注:P < 0.05,说明乔木和灌木的SMA斜率差异显著。Notes: P < 0.05, the SMA slope for tree is significantly different from that of the shrub. 表 5 SLA的系统发育信号
Table 5 Phylogenetic signal of SLA
SLA K P 乔木 Tree 0.21 0.001 灌木 Shrub 0.38 0.037 表 6 遗传距离矩阵和SLA欧式距离矩阵的相关性
Table 6 Correlations between genetic distance matrix and Euclidean distance matrix of SLA
矩阵相关性
Correlation between two matricesr P 乔木 Tree 0.417 0.005 灌木 Shrub 0.165 0.070 表 7 气候因子及系统发育对SLA和TN关系影响的混合模型方差分析
Table 7 Summary of mixed-model ANOVA for the effects of climatic factors and phylogeny on the relationship between SLA and TN
项目 Item 乔木 SLA Tree SLA 灌木 SLA Shrub SLA df P SS/% df P SS/% logTN 1 < 0.001*** 34.76 1 < 0.001*** 21.18 年降水量 Mean annual precipitation 6 < 0.001*** 4.42 1 < 0.001*** 23.54 最冷月均温
Mean temperature of the coldest month3 < 0.001*** 1.08 1 < 0.001*** 2.62 潜在蒸散量 Potential evapotranspiration 5 < 0.001*** 8.79 1 < 0.001*** 5.19 样地 Sample plot 1 < 0.001*** 16.96 7 < 0.001*** 13.87 Mya1谱系组 Mya1 division 1 < 0.001*** 23.46 9 < 0.001*** 16.73 Mya2谱系组 Mya2 division 1 < 0.001*** 1.72 4 0.797 0.76 种 Species 9 0.114 0.56 7 < 0.001*** 3.25 A 6 0.342 0.1 1 0.534 0.14 B 3 0.587 0.03 1 0.248 0.53 C 5 0.321 0.11 1 0.595 0.1 D 1 0.118 0.89 7 0.008** 2.32 E 1 0.004** 0.72 8 0.458 0.89 F 1 0.997 0.01 3 0.879 0.26 G 9 0.047* 0.71 7 0.003** 2.72 残差 Residual 93 5.67 55 5.9 注:A、B、C、D、E、F、G分别表示MAP、MTCM、PET、Plot、Mya1、Mya2和Species对SLA和叶片元素含量间关系斜率的影响。 ***P < 0.001, **P < 0.01,*P < 0.05,. P < 0.1。下同。Notes: A, B, C, D, E, F, G represent the effects of MAP, MTCM, PET, Plot, Mya1, Mya2, and species on the slope of the relationship between SLA and leaf element concentration. *** means P < 0.001, ** means P < 0.01, * means P < 0.05, . means P < 0.1. The same below. 表 8 气候因子及系统发育对SLA和TC关系影响的混合模型方差分析
Table 8 Summary of mixed-model ANOVA for the effects of climatic factors and phylogeny on the relationship between SLA and TC
项目 Item 乔木SLA Tree SLA 灌木SLA Shrub SLA df P SS/% df P SS/% logTC 1 < 0.001*** 20.01 1 < 0.001*** 10.99 年降水量
Mean annual precipitation1 < 0.001*** 0.88 1 < 0.001*** 30.55 最冷月均温
Mean temperature of the coldest month1 < 0.001*** 0.76 1 < 0.001*** 10.17 潜在蒸散量
Potential evapotranspiration1 0.717 0.25 1 < 0.001*** 4.79 样地 Sample plot 9 < 0.001*** 15.85 7 < 0.001*** 16.33 Mya1谱系组 Mya1 division 6 < 0.001*** 53.88 9 < 0.001*** 11.41 Mya2谱系组 Mya2 division 3 < 0.001*** 1.64 4 0.36 2.13 种 Species 5 0.02* 0.63 7 0.008** 2.9 A 1 0.007** 0.43 1 0.133 0.42 B 1 0.19 0.07 1 0.806 0.01 C 1 0.07· 0.15 1 0.084· 0.59 D 9 0.598 0.32 7 0.363 1.01 E 6 0.792 0.62 7 0.206 1.08 F 3 0.268 0.64 2 0.9 0.07 G 5 0.025* 0.6 6 0.027* 2.05 残差 Residual 75 3.27 43 5.51 表 9 气候因子及系统发育对SLA和TP关系影响的混合模型方差分析
Table 9 Summary of mixed-model ANOVA for the effects of climatic factors and phylogeny on the relationship between SLA and TP
项目 Item 乔木 SLA Tree SLA 灌木 SLA Shrub SLA df P SS/% df P SS/% logTP 1 < 0.001*** 25.38 1 < 0.001*** 4.54 年降水量
Mean annual precipitation1 < 0.001*** 7.1 1 < 0.001*** 32.41 最冷月均温
Mean temperature of the coldest month1 < 0.001*** 1.53 1 < 0.001*** 8.04 潜在蒸散量
Potential evapotranspiration1 0.672 0.25 1 < 0.001*** 3.38 样地 Sample plot 9 < 0.001*** 11.81 7 < 0.001*** 15.35 Mya1谱系组 Mya1 division 6 < 0.001*** 44.46 9 < 0.001*** 16.82 Mya2谱系组 Mya2 division 5 < 0.001*** 1.5 4 0.7 1.19 种 Species 5 0.018* 0.58 7 < 0.001*** 3.73 A 1 0.181 0.35 1 0.497 0.19 B 1 0.58 0.05 1 0.93 0.002 C 1 0.72 0.02 1 < 0.001*** 1.84 D 9 < 0.001*** 1.49 7 < 0.001*** 2.56 E 6 0.385 0.29 9 0.065· 1.9 F 5 0.588 0.18 4 0.957 0.16 G 5 0.36 0.22 6 0.014* 1.66 残差 Residual 118 4.78 65 6.21 -
[1] Smith W K, Vogelmann T C, Delucia E H, et al. Leaf form and photosynthesis[J]. BioScience, 1997, 47(11): 785−793. doi: 10.2307/1313100
[2] Wilson P J, Thompson K E N, Hodgson J G. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies[J]. New Phytologist, 1999, 143(1): 155−162. doi: 10.1046/j.1469-8137.1999.00427.x
[3] 董莉莉, 刘世荣, 史作民, 等. 中国南北样带上栲属树种叶功能性状与环境因子的关系[J]. 林业科学研究, 2009, 22(4):463−469. doi: 10.3321/j.issn:1001-1498.2009.04.001 Dong L L, Liu S R, Shi Z M, et al. Relationships between leaf traits of Castanopsis species and the environmental factors in the North-South transect of eastern China[J]. Forest Research, 2009, 22(4): 463−469. doi: 10.3321/j.issn:1001-1498.2009.04.001
[4] 连政华, 张春雨, 程艳霞, 等. 中国东北部典型树种功能性状地理变异规律研究[J]. 北京林业大学学报, 2019, 41(3):42−48. Lian Z H, Zhang C Y, Cheng Y X, et al. Geographical variations of functional traits of typical tree species in northeastern China[J]. Journal of Beijing Forestry University, 2019, 41(3): 42−48.
[5] 曹科, 饶米德, 余建中, 等. 古田山木本植物功能性状的系统发育信号及其对群落结构的影响[J]. 生物多样性, 2013, 21(5):564−571. Cao K, Rao M D, Yu J Z, et al. The phylogenetic signal of functional traits and their effects on community structure in an evergreen broad-leaved forest[J]. Biodiversity Science, 2013, 21(5): 564−571.
[6] 邓蕾, 王鸿喆, 上官周平, 等. 水蚀风蚀交错区柠条锦鸡儿叶片比叶面积和营养元素变化动态[J]. 生态学报, 2010, 30(18):4889−4897. Deng L, Wang H Z, Shangguan Z P, et al. Variations of specific leaf area and nutrients of Chinese caragana in the Loess Plateau region suffering both wind and water erosions[J]. Acta Ecologica Sinica, 2010, 30(18): 4889−4897.
[7] 徐朝斌, 钟全林, 程栋梁, 等. 基于地理种源的刨花楠苗木比叶面积与叶片化学计量学关系[J]. 生态学报, 2015, 35(19):6507−6515. Xu C B, Zhong Q L, Cheng D L, et al. Variation in relationships between SLA and leaf C, N, P stoichiometry in Machilus pauhoi among locations[J]. Acta Ecologica Sinica, 2015, 35(19): 6507−6515.
[8] 杨美华. 长白山的气候特征及北坡垂直气候带[J]. 气象学报, 1981, 39(3):311−319. Yang M H. The climatic features of Changbaishan and its vertical climate zone on the northern slop[J]. Acta Meteorologica Sinica, 1981, 39(3): 311−319.
[9] Kembel S W, Cahill J F. Independent evolution of leaf and root traits within and among temperate grassland plant communities [J/OL]. PLoS one, 2011, 6(6): e19992 [2018−11−20] (2011−06−08). https://doi.org/10.1371/journal.pone.0019992.
[10] Zhang M, Ji C, Zhu J, et al. Comparison of wood physical and mechanical traits between major gymnosperm and angiosperm tree species in China[J]. Wood Science and Technology, 2017, 51(6): 1405−1419. doi: 10.1007/s00226-017-0954-1
[11] Webb C O, Donoghue M J. Phylomatic: tree assembly for applied phylogenetics[J]. Molecular Ecology Notes, 2005, 5(1): 181−183. doi: 10.1111/j.1471-8286.2004.00829.x
[12] Zhang S B, Slik J W F, Zhang J L, et al. Spatial patterns of wood traits in China are controlled by phylogeny and the environment[J]. Global Ecology and Biogeography, 2011, 20(2): 241−250. doi: 10.1111/j.1466-8238.2010.00582.x
[13] Kress W J, Erickson D L, Jones F A, et al. Plant DNA barcodes and a community phylogeny of a tropical forest dynamics plot in Panama[J]. Proceedings of the National Academy of Sciences, 2009, 106(44): 18621−18626. doi: 10.1073/pnas.0909820106
[14] Group C P W, Hollingsworth P M, Forrest L L, et al. A DNA barcode for land plants[J]. Proceedings of the National Academy of Sciences, 2009, 106(31): 12794−12797. doi: 10.1073/pnas.0905845106
[15] 裴男才, 张金龙, 米湘成, 等. 植物DNA条形码促进系统发育群落生态学发展[J]. 生物多样性, 2011, 19(3):284−294. Pei N C, Zhang J L, Mi X C, et al. Plant DNA barcodes promote the development of phylogenetic community ecology[J]. Biodiversity Science, 2011, 19(3): 284−294.
[16] Reich P B, Oleksyn J. Global patterns of plant leaf N and P in relation to temperature and latitude[J]. Proceedings of the National Academy of Sciences, 2004, 101(30): 11001−11006. doi: 10.1073/pnas.0403588101
[17] Elser J J, Bracken M E S, Cleland E E, et al. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems[J]. Ecology Letters, 2007, 10(12): 1135−1142. doi: 10.1111/j.1461-0248.2007.01113.x
[18] Wright I J, Reich P B, Westoby M. Strategy shifts in leaf physiology, structure and nutrient content between species of high- and low-rainfall and high- and low-nutrient habitats[J]. Functional Ecology, 2001, 15(4): 423−434. doi: 10.1046/j.0269-8463.2001.00542.x
[19] 么旭阳, 胡耀升, 刘艳红. 长白山阔叶红松林不同群落类型的植物功能性状与功能多样性[J]. 西北农林科技大学学报(自然科学版), 2014, 42(3):77−84. Yao X Y, Hu Y S, Liu Y H. Plant functional traits and functional diversities of different communities in broad-leaved Korean pine forests in the Changbai Mountain[J]. Journal of Northwest Sci-Tech University of Agriculture and Forestry(Natural Science Edition), 2014, 42(3): 77−84.
[20] 杨蕾, 孙晗, 樊艳文, 等. 长白山木本植物叶片氮磷含量的海拔梯度格局及影响因子[J]. 植物生态学报, 2017, 41(12):1228−1238. doi: 10.17521/cjpe.2017.0115 Yang L, Sun H, Fan Y W, et al. Changes in leaf nitrogen and phosphorus stoichiometry of woody plants along an altitudinal gradient in Changbai Mountain, China[J]. Chinese Journal of Plant Ecology, 2017, 41(12): 1228−1238. doi: 10.17521/cjpe.2017.0115
[21] 姜沛沛, 曹扬, 陈云明. 陕西省森林群落乔灌草叶片和凋落物C、N、P生态化学计量特征[J]. 应用生态学报, 2016, 27(2):365−372. Jiang P P, Cao Y, Chen Y M. C, N, P stoichiometric characteristics of tree, shrub, herb leaves and litter in forest community of Shaanxi Province, China[J]. Chinese Journal of Applied Ecology, 2016, 27(2): 365−372.
[22] 戴志聪, 杜道林, 司春灿, 等. 用扫描仪及Image J软件精确测量叶片形态数量特征的方法[J]. 广西植物, 2009, 29(3):342−347. doi: 10.3969/j.issn.1000-3142.2009.03.013 Dai Z Q, Du D L, Si C C, et al. A method to exactly measure the morphological quantity of leaf using Scanner and Image J Software[J]. Guihaia, 2009, 29(3): 342−347. doi: 10.3969/j.issn.1000-3142.2009.03.013
[23] 王维华. 红松针叶面积的测定[J]. 辽宁林业科技, 1985(2):22−24. Wang W H. Methods to measure the leaf area of Pinus koraiensis[J]. Liaoning Forestry Science and Technology, 1985(2): 22−24.
[24] 方精云. 地理要素对我国温度分布影响的数量评价[J]. 生态学报, 1992, 12(2):97−104. doi: 10.3321/j.issn:1000-0933.1992.02.006 Fang J Y. Study on the geographic elements affecting temperature distribution in China[J]. Acta Ecologica Sinica, 1992, 12(2): 97−104. doi: 10.3321/j.issn:1000-0933.1992.02.006
[25] Wang X P, Fang J Y, Zhu B. Forest biomass and root-shoot allocation in northeast China[J]. Forest Ecology and Management, 2008, 255(12): 4007−4020. doi: 10.1016/j.foreco.2008.03.055
[26] Barrufol M, Schmid B, Bruelheide H, et al. Biodiversity promotes tree growth during succession in subtropical forest[J/OL]. PLoS one, 2013, 8(11): e81246 (2013−09−26) [2018−11−02]. https://doi.org/10.1371/journal.pone.0081246.
[27] Farris J S, Källersjö M, Kluge A G, et al. Testing significance of incongruence[J]. Cladistics, 1994, 10(3): 315−319. doi: 10.1111/j.1096-0031.1994.tb00181.x
[28] Nguyen L T, Schmidt H A, Von Haeseler A, et al. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies[J]. Molecular Biology and Evolution, 2015, 32(1): 268−274.
[29] Sanderson M J. r8s: inferring absolute rates of molecular evolution and divergence times in the absence of a molecular clock[J]. Bioinformatics, 2003, 19(2): 301−302. doi: 10.1093/bioinformatics/19.2.301
[30] He J S, Wang X P, Flynn D F B, et al. Taxonomic, phylogenetic, and environmental trade-offs between leaf productivity and persistence[J]. Ecology, 2009, 90(10): 2779−2791. doi: 10.1890/08-1126.1
[31] Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets[J]. Molecular Biology and Evolution, 2016, 33(7): 1870−1874. doi: 10.1093/molbev/msw054
[32] Kembel S W, Cowan P D, Helmus M R, et al. Picante: R tools for integrating phylogenies and ecology[J]. Bioinformatics, 2010, 26(11): 1463−1464. doi: 10.1093/bioinformatics/btq166
[33] Sun H, Wang X P, Fan Y W, et al. Effects of biophysical constraints, climate and phylogeny on forest shrub allometries along an altitudinal gradient in Northeast China[J/OL]. Scientific Reports, 2017, 7: 43769, https://doi.org/10.1038/srep43769.
[34] Wright I J, Reich P B, Westoby M, et al. The worldwide leaf economics spectrum[J]. Nature, 2004, 428: 821−827. doi: 10.1038/nature02403
[35] Wright I J, Westoby M. Leaves at low versus high rainfall: coordination of structure, lifespan and physiology[J]. New Phytologist, 2002, 155(3): 403−416. doi: 10.1046/j.1469-8137.2002.00479.x
[36] Reich P B, Walters M B, Ellsworth D S. From tropics to tundra: Global convergence in plant functioning[J]. Proceedings of the National Academy of Sciences, 1997, 94(25): 13730−13734. doi: 10.1073/pnas.94.25.13730
[37] Milla R, Reich P B. Multi-trait interactions, not phylogeny, fine-tune leaf size reduction with increasing altitude[J]. Annals of Botany, 2011, 107(3): 455−465. doi: 10.1093/aob/mcq261
[38] 房帅, 原作强, 蔺菲, 等. 长白山阔叶红松林木本植物系统发育与功能性状结构[J]. 科学通报, 2014, 59(24):2342−2348. doi: 10.1360/N972014-00291 Fang S, Yuan Z Q, Lin F, et al. Functional and phylogenetic structures of woody plants in broad-leaved Korean pine mixed forest in Changbai Mountains, Jilin, China[J]. Chinese Science Bulletin, 2014, 59(24): 2342−2348. doi: 10.1360/N972014-00291
[39] Jefferson L V, Pennacchio M. The impact of shade on establishment of shrubs adapted to the high light irradiation of semi-arid environments[J]. Journal of Arid Environments, 2005, 63(4): 706−716. doi: 10.1016/j.jaridenv.2005.04.004
[40] 田杰, 王庆伟, 于大炮, 等. 长白山北坡气温的垂直变化[J]. 干旱区资源与环境, 2013, 27(4):65−69. Tian J, Wang Q W, Yu D P, et al. Air temperature variation along altitudinal gradient on the northern slope of Mt. Changbai, China[J]. Journal of Arid Land Resources and Environment, 2013, 27(4): 65−69.
[41] Treml V, Hejda T, Kašpar J. Differences in growth between shrubs and trees: How does the stature of woody plants influence their ability to thrive in cold regions?[J]. Agricultural and Forest Meteorology, 2019, 271: 54−63. doi: 10.1016/j.agrformet.2019.02.036
[42] Kergunteuil A, Descombes P, Glauser G, et al. Plant physical and chemical defence variation along elevation gradients: a functional trait-based approach[J]. Oecologia, 2018, 187(2): 561−571. doi: 10.1007/s00442-018-4162-y
[43] Poorter H, De Jong R O B. A comparison of specific leaf area, chemical composition and leaf construction costs of field plants from 15 habitats differing in productivity[J]. New Phytologist, 1999, 143(1): 163−176. doi: 10.1046/j.1469-8137.1999.00428.x
[44] Hoch G, Popp M, Körner C. Altitudinal increase of mobile carbon pools in Pinus cembra suggests sink limitation of growth at the Swiss treeline[J]. Oikos, 2002, 98(3): 361−374. doi: 10.1034/j.1600-0706.2002.980301.x
[45] Hoffmann W A, Franco A C, Moreira M Z, et al. Specific leaf area explains differences in leaf traits between congeneric savanna and forest trees[J]. Functional Ecology, 2005, 19(6): 932−940. doi: 10.1111/j.1365-2435.2005.01045.x
[46] Lambers H, Chapin Ⅲ F S, Pons T L. Plant Physiological Ecology[M]. New York: Springer Science & Business Media, 2008.
[47] 李合生. 现代植物生理学[M]. 北京: 高等教育出版社, 2006. Li H S. Modern plant physiology[M]. Beijing: Higher Education Press, 2006.
[48] Yang S Y, Huang T K, Kuo H F, et al. Role of vacuoles in phosphorus storage and remobilization[J]. Journal of Experimental Botany, 2017, 68(12): 3045−3055.
[49] Han W X, Fang J Y, Reich P B, et al. Biogeography and variability of eleven mineral elements in plant leaves across gradients of climate, soil and plant functional type in China[J]. Ecology Letters, 2011, 14(8): 788−796. doi: 10.1111/j.1461-0248.2011.01641.x
[50] 贺金生, 韩兴国. 生态化学计量学: 探索从个体到生态系统的统一化理论[J]. 植物生态学报, 2010, 34(1):2−6. doi: 10.3773/j.issn.1005-264x.2010.01.002 He J S, Han X G. Ecological stoichiometry: searching for unifying principles from individuals to ecosystems[J]. Chinese Journal of Plant Ecology, 2010, 34(1): 2−6. doi: 10.3773/j.issn.1005-264x.2010.01.002
-
期刊类型引用(2)
1. 王博,杨雪清,蒋春颖,赖光辉,陈锋,刘晓东. 北京山区森林火灾蔓延风险评估. 生态学报. 2025(02): 813-821 .  百度学术
百度学术
2. 律江,贾玮,刘洋,刘阳. 城市与森林融合的国有林场森林防火地面防控新范式探索——以北京市西山试验林场为例. 森林防火. 2024(03): 42-45 .  百度学术
百度学术
其他类型引用(0)



 下载:
下载: