Relationship between leaf functional trait variation of Cotinus coggygria seedling and location geographical-climatic factors under drought stress
-
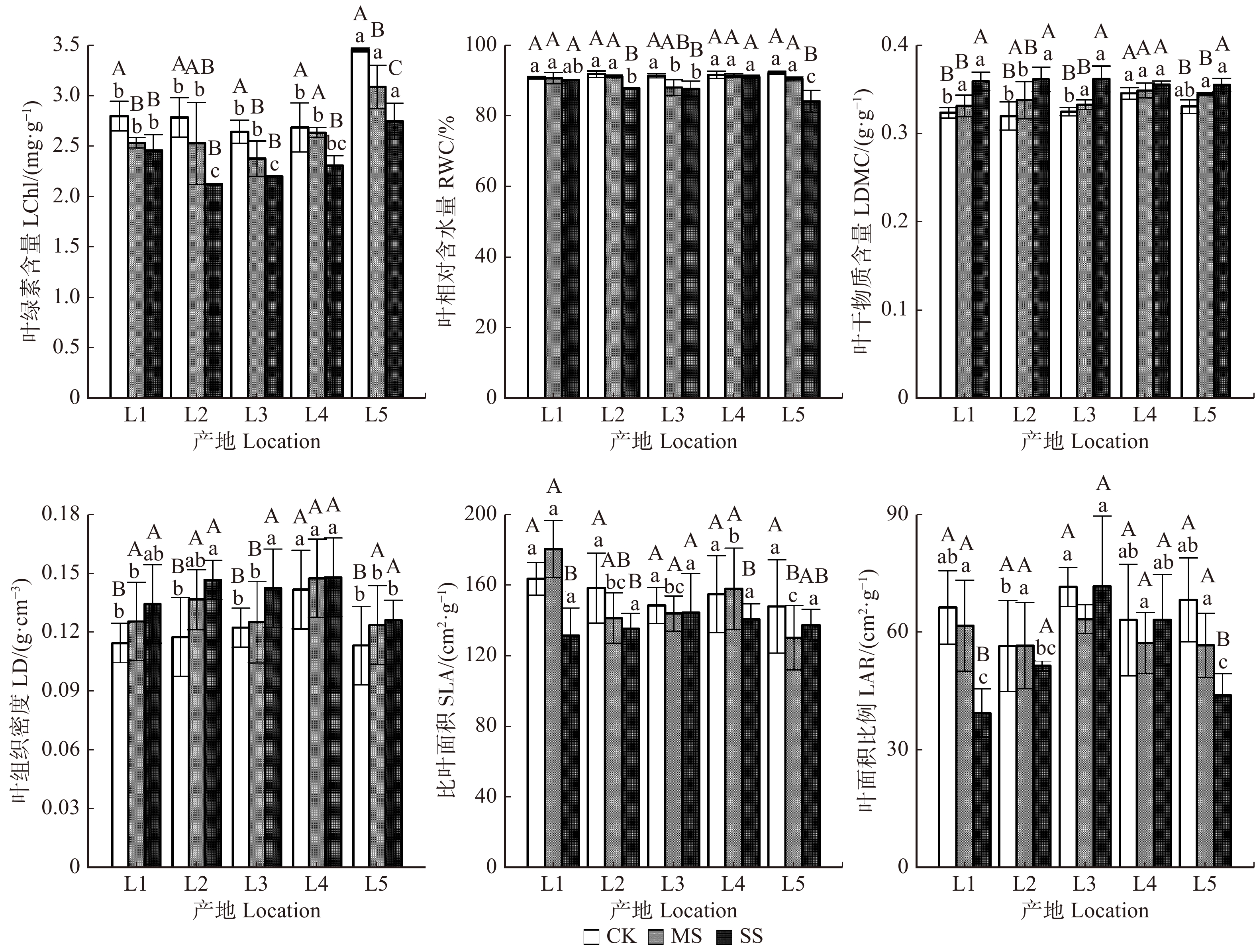
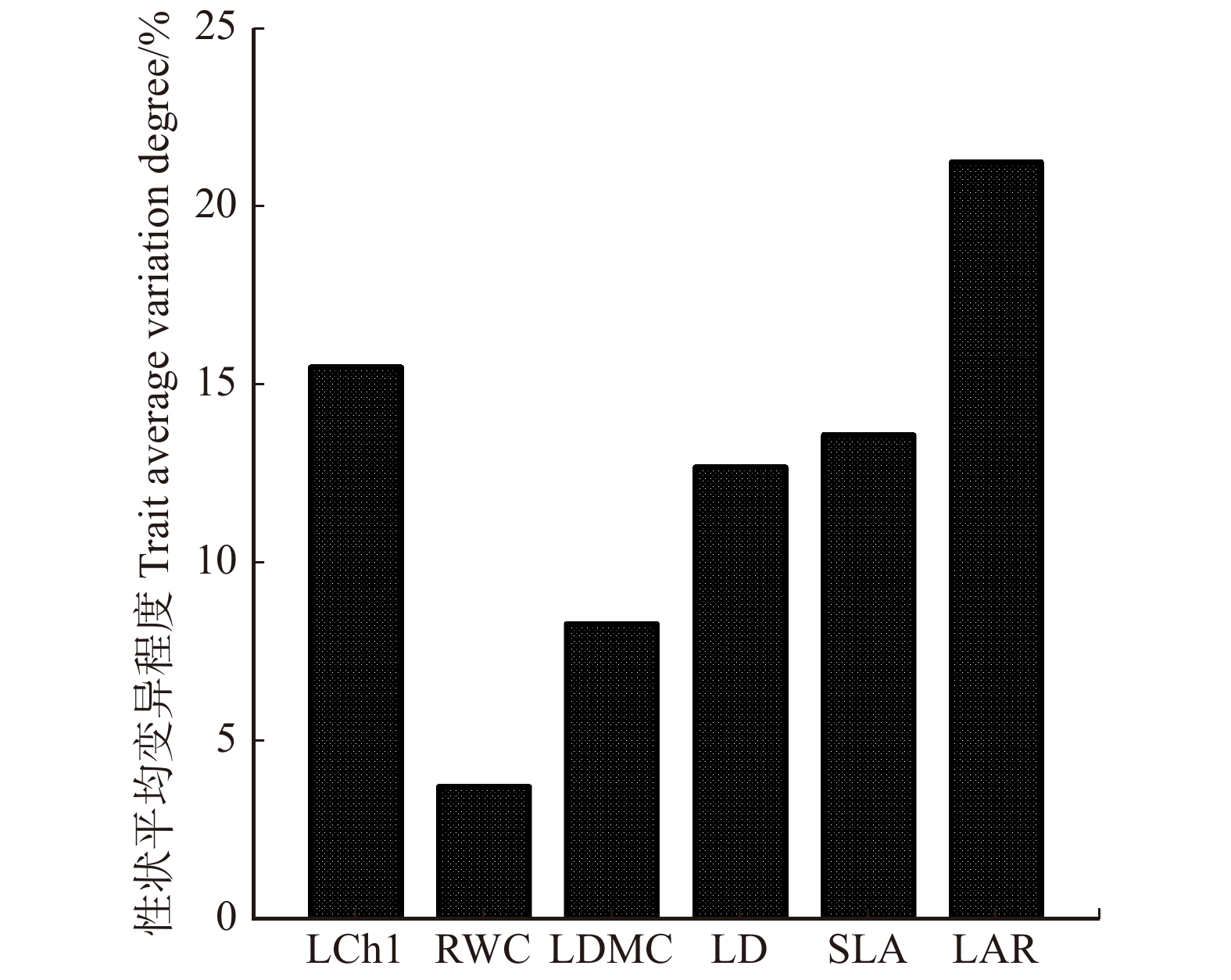
摘要:目的分析持续干旱胁迫环境中不同产地黄栌幼苗叶功能性状的变异规律及差异,并探究产地地理−气候因子对叶功能性状变异的影响。方法采用田间模拟试验方法,选取来自5个产地的黄栌1年生幼苗作为供试材料,设置对照(CK,土壤田间持水量的75% ~ 80%)、中度胁迫(MS,土壤田间持水量的55% ~ 65%)和重度胁迫(SS,土壤田间持水量的35% ~ 45%)3个土壤水分含量梯度,分析干旱胁迫、产地及其二者的交互作用对叶功能性状的影响,并结合主成分分析(PCA)和冗余分析(RDA)分别研究产地分布与地理−气候因子的关系以及产地地理−气候因子对叶功能性状变异程度(TVD)的影响。结果(1)干旱胁迫对幼苗叶功能性状均具有显著影响。其中,SS环境中,叶绿素含量(LChl)、叶相对含水量(RWC)、比叶面积(SLA)和叶片面积比例(LAR)分别比对照低17.61%(P < 0.001)、3.71%(P < 0.001)、10.89%(P = 0.002)和17.22%(P = 0.001),叶干物质含量(LDMC)、叶组织密度(LD)分别比对照高9.04%(P < 0.001)和14.52%(P = 0.009)。(2)黄栌叶功能性状之间的相关性在干旱胁迫环境中具有增强的趋势。其中,SLA与LDMC和LD均呈极显著(P < 0.01)的负相关关系,LDMC和LD之间具有极显著(P < 0.01)的正相关关系,RWC与LChl(P < 0.01)、SLA(P < 0.01)和LAR(P < 0.05)呈极显著或显著的正相关关系。(3)干旱胁迫环境中不同产地叶功能性状具有显著差异。MS处理时,SLA(P = 0.002)、LChl(P = 0.025)和LD(P = 0.026)在产地间具有显著差异;SS处理时,LChl(P < 0.001)、LAR(P < 0.001)和RWC(P = 0.005)存在显著的产地差异。(4)来自北京延庆区幼苗的叶功能性状平均变异程度(所有性状变异程度的平均值)最大,为17.57%,而来自山西运城绛县幼苗的最小,为6.97%。(5)经RDA筛选,最干燥月份降水量(DMP,P = 0.002)、生长季月降水量平均差(GSPD,P = 0.008)、最热月份的最高气温(WMT,P = 0.016)和年平均降水量(ANP,P = 0.036)对幼苗TVD影响显著。其中,DMP与所有性状的变异程度之间均具有不同程度的负相关关系,但与LDMC和LD变异程度之间的负相关性更为显著,GSPD和ANP与SLA和LAR变异程度的负相关性较为显著,WMT与LChl变异程度的正相关性极为密切。结论黄栌叶功能性状在不同干旱胁迫环境中、不同产地间均具有显著差异。产地气候 (尤其是DMP、GSPD、WMT和ANP)是导致干旱逆境中不同产地黄栌叶功能性状发生遗传变异的重要原因。5产地中,山西运城绛县的DMP最高,GSPD和ANP较为适宜,WMT较低,且来自该产地的黄栌叶功能性状在干旱胁迫中的平均变异程度最小,因此较适合引种至华北地区的干旱区域。Abstract:ObjectiveThis study aims to identify leaf functional trait variation patterns and differences of Cotinus coggygria seedlings from different locations and to analyse the influences of different geographic-climatic factors on leaf functional trait variations under continuous drought environment.MethodA standard continuous drought stress experiment was carried out using one-year-old C. coggygria seedlings from five different locations within China. Three levels of water regimes were set: control (CK, 75% ~ 80% of soil field capacity), moderate stress (MS, 55% ~ 65% of soil field capacity) and severe stress (SS, 35% ~ 45% of soil field capacity). ANOVA was used to identify the effects of drought, location and their interaction on leaf functional traits. On the other hand, the principal component analysis (PCA) and the redundancy analysis (RDA) were used to measure the relationship between location distribution of the species and the geographical-climatic factors and the influences of geographical-climatic conditions on leaf functional trait variation degree (TVD), respectively.Result(1) Drought stress had significant effects on all the leaf functional traits. Seedlings under SS had lower leaf function traits in terms of leaf chlorophyll content (LChl, 17.61%, P < 0.001), relative water content (RWC, 3.71%, P < 0.001), specific leaf area (SLA, 10.89%, P = 0.002), and leaf area ratio (LAR, 17.22%, P = 0.001) compared to the seedlings under CK. However, seedlings under SS had higher leaf dry matter content (LDMC) and leaf density (LD) than control by 9.04% (P < 0.001) and 14.52% (P = 0.009), respectively. (2) Correlations among leaf functional traits became stronger in drought environment, which showed that SLA had significantly (P < 0.01) negative links with LDMC and LD, LDMC and LD had a significant (P < 0.01) and positive relation, and RWC had significantly positive correlations with LChl (P < 0.01), SLA (P < 0.01) and LAR (P < 0.05). (3) The leaf functional traits showed significant differences among C. coggygria locations under drought treatments. SLA (P = 0.002), LChl (P = 0.025) and LD (P = 0.026) were significantly different under MS treatment, and LChl (P < 0.001), LAR (P < 0.001) and RWC (P = 0.005) were significantly different under SS treatment. (4) Among the five different locations, C. coggygria seedlings from Yanqing County in Beijing had the highest average trait variation degree (the mean values of all trait variation degrees) of 17.57%, while the lowest was from Jiang County of Yuncheng City in Shanxi Province of 6.97%. (5) After the screening of RDA, precipitation of the driest month (DMP, P = 0.002), growing season mean monthly precipitation difference (GSPD, P = 0.008), Max. temperature of the warmest month (WMT, P = 0.016) and average annual precipitation (ANP, P = 0.036) had significant effects on leaf functional trait variation degree. Particularly, DMP had negative relationships with all trait variation degree, but had more significantly negative correlations with the variation degree of LDMC and LD. GSPD and ANP had significantly negative correlationss with the variation degree of SLA and LAR. WMT was closer to the variation degree of LChl.ConclusionSignificant differences were found for leaf functional traits of C. coggygria among different drought treatments and different locations. The local climate (especially DMP, GSPD, WMT and ANP) was the main cause of leaf functional trait variation of C. coggygria from different locations under drought stress. Among seedlings from the five locations explored in our study, seedlings from Jiang County of Yuncheng City in Shanxi Province were more suitable to be introduced to the arid areas in northern China, as a result of a relatively high DMP, a proper GSPD and ANP, a relatively low WMT, and a low average leaf functional trait variation degree under drought stress.
-
Keywords:
- Cotinus coggygria /
- leaf functional trait /
- geographical-climatic factor /
- drought stress /
- location
-
赤霉素(GAs)是一种重要植物激素,在植物整个生命周期中对生长发育的各个阶段具有广泛的调控作用[1-2]。GAs通过促进细胞分裂和细胞伸长[3-6]进而促进茎部节间的伸长生长。李哲馨[7]的研究表明GAs在烟草茎部形成层发挥重要作用,对植株生长和木质部发育具有明显的促进作用。因此,GAs代谢调控在速生林林木育种中具有重要的应用潜力;然而,以往的研究都是基于施加外源活性GAs或全株组成型调控GAs含量的分析,并没有考虑活性GAs在活体内的运输特性对研究结果的影响,有研究表明移动的GAs对茎的次生生长与木质部形成层的发育具有直接的促进作用[8]。另外,由于GAs参与调控多个发育过程,特异下调茎部GAs含量在延缓茎部生长的同时,也会抑制不定根的发生[9],影响根尖的径向生长[10]。有研究表明茎部GAs在低温胁迫的响应过程中具有反馈调控作用[11-12],因此,开展对茎部特异性GAs含量的调控研究,为GAs代谢调控植物生长发育在林木育种中的实际应用提供理论基础。
形成层位于木质部和韧皮部之间,是一种重要的分生组织,细胞分裂旺盛,向外分裂形成新的韧皮部细胞,向茎的中轴方向分裂形成新的木质部细胞,大量调控木材合成的基因在此过程中表达[13]。研究发现在形成层细胞分裂时,GAs加快了形成层细胞的分裂速度,促进了细胞的伸长,从而促进导管及筛管的伸长[14]。此后的研究中进一步证实了形成层GAs对植株生长发育有促进作用,但在外源施加GAs时,尚不能排除由于植物组织损伤而造成植株自身激素含量变化的误差,并且植物某一发育时间或某一组织中的外源激素含量的测定也难以精准,给实验带来很多不确定性[15]。因此,特异性降低植物形成层GAs含量对植物生长发育的调控作用有待于进一步深入研究。
已有研究表明,在植物体内自由运输的可能并不是活性GAs,而是其前体GA12[16]。植物嫁接是研究活性物质长距离运输特性的有效手段[17]。本实验通过嫁接不同激素含量的转基因植株及野生型WT植株再配合外施赤霉素进行实验分析,验证活性GAs在烟草中的运输机制,更深入地探讨组织特异性GAs含量对植株生长发育的调控作用,对今后GAs调控植物各组织发育的研究具有重要意义,为林木速生优质新品种定向培育策略的制定提供理论基础。
1. 材料与方法
1.1 实验材料
以本实验室遗传转化获得的烟草转基因株系LMX5:: PtGA2ox1、35S:: PtGA20ox、35S:: PtGA2ox1及野生型烟草为研究材料(其中35S:: PtGA20ox、35S:: PtGA2ox1来自林木遗传育种实验室的保存材料),分别记作L:G2、35:G20、35:G2与WT。PtGA2ox1基因克隆于毛白杨,转基因受体为野生型烟草W38株系(Nicotiana tabacum cultivar Wisconsin 38)。所有植物材料均在不含任何激素的MS培养基上,组培室温度为25 ℃,光照周期为16 h光照/8 h黑暗,将继代培养2月苗龄的生根苗移栽到温室,在30 ℃自然光照下培养。
1.2 烟草形成层赤霉素特异性调控转基因株系的获得
用毛白杨的成熟叶片提取高质量RNA,反转录获得cDNA,再以cDNA为模板克隆赤霉素代谢中的关键基因PtGA2ox1,构建表达载体LMX5::PtGA2ox1,并转化表达载体到农杆菌中。通过农杆菌介导的转基因技术获得以木质部形成层特异性启动子(LMX5)启动的烟草转基因株系L:G2[18]。
1.3 基因表达分析及GAs含量的测定
分别提取转基因烟草L:G2、35:G2及WT植株茎部、叶片和根部组织的总RNA,反转录得到cDNA,根据差异片段序列设计引物,半定量RT-PCR反应条件:95 ℃变性3 min,随后进行以下40个循环:95 ℃变性3 s,60 ℃退火20 s,72 ℃延伸30 s。以Actin为内标基因,扩增引物序列为FW:5′-CACAAGCCAGCACTTCAACAG-3′,RV:5′-ATGCCTTAACCAGGAGGTGC-3′。将每个扩增反应做3次重复实验,每次均设置阴性对照,并利用软件Bio-Rad CFXManager 2.0读取相关数据。
分别取组织培养30 d的35:G20、35:G2、L:G2与WT植株的叶、茎、根3个部位的样品各3 g,将其放在研钵中进行研磨,用LC-MS(液相色谱−质谱联用仪)对样品进行测定分析[19],每个样本进行3次重复实验。
1.4 烟草的微嫁接
以35:G2烟草转基因株系的顶芽作接穗、35:G20植株茎杆的一段作砧木,从砧木的横截面中心向下垂直的劈开切接口,使劈口长度与接穗的削面相吻合,再用锡箔纸将接穗与砧木紧密包裹,得到以35:G2为接穗,35:G20为砧木的独立嫁接苗(以下写作为35:G2//35:G20),以同样方法分别获得嫁接苗35:G20//35:G2、35:G2//WT以及WT//WT。
1.5 植株各部分生长性状的测定
挑选培养45 d的无菌生根苗L:G2、35:G2、35:G20与WT,分别对其茎部进行切片,用0.5%番红染液染色后对木质部进行显微观察并拍照。
将烟草转基因株系L:G2、35:G2和35:G20的顶芽扦插在无激素的MS培养基上,设置WT为对照,培养20 d后对各组的生根情况进行观察,再从4组株系中各选取30株,对其不定根的长度进行测量,计算平均值后与对照组一同进行t检验。
2. 结果与分析
2.1 转基因株系的获得及基因表达分析
通过农杆菌介导的转基因技术获得了以木质部形成层特异性启动子LMX5介导的烟草转基因株系L:G2,提取植株的DNA进行PCR扩增反应进行初步鉴定,成功获得21个转基因株系,与野生型WT相比,转基因植株(图1A)生长矮小,不定根数目减少,差异明显。
![]() 图 1 不同烟草植株及基因表达水平A是不同烟草植株,标尺长度为1 cm;B是转基因株系各组织中PtGA2ox1基因的表达水平;C是转基因株系不同组织中烟草NtGA2ox1基因本底表达水平,将35:G2植株中的PtGA2ox1基因的表达量设置为1。A is different tobacco plant with a scale length of 1 cm; B is the expression level of PtGA2ox1 gene in each tissue of transgenic lines; C is the background expression level of tobacco NtGA2ox1 gene in different tissues of transgenic lines, which will be in 35:G2 plants, the expression level of the PtGA2ox1 gene was set to 1.Figure 1. Different tobacco plants and gene expression levels
图 1 不同烟草植株及基因表达水平A是不同烟草植株,标尺长度为1 cm;B是转基因株系各组织中PtGA2ox1基因的表达水平;C是转基因株系不同组织中烟草NtGA2ox1基因本底表达水平,将35:G2植株中的PtGA2ox1基因的表达量设置为1。A is different tobacco plant with a scale length of 1 cm; B is the expression level of PtGA2ox1 gene in each tissue of transgenic lines; C is the background expression level of tobacco NtGA2ox1 gene in different tissues of transgenic lines, which will be in 35:G2 plants, the expression level of the PtGA2ox1 gene was set to 1.Figure 1. Different tobacco plants and gene expression levels通过测定转基因烟草L:G2、35:G2外源基因的表达水平(图1B、1C),发现L:G2、35:G2转基因植株的表达模式不同,PtGA2ox1转录水平只在L:G2的茎部出现了明显的上升趋势,而叶片和根部的上升趋势不明显。通过测定转基因烟草的叶片、根部及茎部中NtGA2ox1基因的本底表达水平,发现转基因烟草株系L:G2在叶片和根部受自身调控的NtGA2ox1基因表达水平与野生型WT对照比并无明显变化,而茎部NtGA2ox1基因的表达水平和野生型WT相比却明显降低;同时发现转基因烟草株系35:G2各组织中的NtGA2ox1基因表达水平与WT相比均有明显的下降。
在烟草中,主要活性赤霉素GA1在L:G2的茎部含量明显低于野生型WT植株,而非活性的GA8在茎部的含量与WT相比有显著的提高(图2);而转基因株系35:G20与WT相比,根、茎以及叶片内活性GAs(主要是GA1)含量显著提高,即植株整体GAs含量上调(图2);转基因植株35:G2茎、叶部分的活性GAs含量均有显著的下调,而非活性GAs含量增多,植株整体GAs含量下调,可能是GAs含量下降负反馈调节导致活性GA2-氧化酶含量下降 (图2)。
2.2 活性GAs在烟草茎中的运输特性
对35:G20//35:G2、35:G2//35:G20、及WT//WT 3组嫁接苗进行观察,发现接穗和砧木的表型性状与其各自基因型植株的性状并无较大差异(图3)。表明35:G20植株体内产生的大量活性GAs无法在嫁接植株中自由转运到35:G2中,而35:G2植株中的GA2-氧化酶也无法转化35:G20中的大量活性GAs;但培养在添加有GA4+7培养基中的WT//WT嫁接植株,相同生长时间内接穗和砧木的性状都受GAs影响,与35:G20表型相似,均表现为生长迅速,节间和叶片快速伸长,叶片颜色呈浅绿色细长的状态。
![]() 图 3 不同基因型组成的嫁接苗和不同基因型的烟草植株A.普通MS培养基中的WT//WT嫁接植株; B.添加有GA4+7的WT//WT嫁接植株; C.普通MS培养基中的35:G2//35:G20嫁接植株; D.普通MS培养基中的35:G20//35:G2嫁接植株; E.不同基因型的烟草植株。箭头代表嫁接处;A ~ D标尺长度为2 cm;E标尺长度为1 cm。The E scale is 1 cm in length; A,WT//WT grafted plants in normal MS medium; B,WT//WT grafted plants with GA4+7 were added; C, G2//35: G20 grafted plants in normal MS medium; D, 35:G20//35:G2 grafted plants in normal MS medium; E,different genotypes of tobacco plants. The arrow represents the graft; the A−D scale is 2 cm in length.Figure 3. Grafted seedlings of different genotypes and tobacco plants of different genotypes
图 3 不同基因型组成的嫁接苗和不同基因型的烟草植株A.普通MS培养基中的WT//WT嫁接植株; B.添加有GA4+7的WT//WT嫁接植株; C.普通MS培养基中的35:G2//35:G20嫁接植株; D.普通MS培养基中的35:G20//35:G2嫁接植株; E.不同基因型的烟草植株。箭头代表嫁接处;A ~ D标尺长度为2 cm;E标尺长度为1 cm。The E scale is 1 cm in length; A,WT//WT grafted plants in normal MS medium; B,WT//WT grafted plants with GA4+7 were added; C, G2//35: G20 grafted plants in normal MS medium; D, 35:G20//35:G2 grafted plants in normal MS medium; E,different genotypes of tobacco plants. The arrow represents the graft; the A−D scale is 2 cm in length.Figure 3. Grafted seedlings of different genotypes and tobacco plants of different genotypes2.3 下调烟草形成层GAs对茎部木质部生长发育的影响
通过转基因方法上调GAs含量促进植株(35:G20)生长,而下调GA含量的植株(35:G2、L:G2)生长缓慢[7],且下调植株整体GAs含量(35:G2)与特异下调形成层中的GAs含量对植株(L:G2)木质部发育的影响程度相似,说明植株GAs含量下降会使木质部的生长发育变延缓,植株的伸长生长也会受到抑制。通过茎部切片观察(图4),发现上调植株内源GAs含量(35:G20),木质部成熟提前,细胞排列整齐紧密且层数增加;下调GAs含量(35:G2、L:G2),木质部分化滞后,成熟缓慢,依据染色区域的大小发现同时期茎部相同位置的初生木质部多于WT,且细胞排列疏松不规则(图4)。转基因植株35:G2与L:G2无论是茎顶部亦或是茎基部组织中木质部的形成与发育的滞后程度、木质部细胞的排列方式等都极为相似,且下调木质部形成层的GAs含量可以达到下调植株整体GAs含量对木质部发育的影响,说明GAs影响植株木质部生长发育主要是在其形成层中发挥作用,形成层中的GAs含量对植株木质部的生长发育起关键作用。
2.4 下调烟草形成层GAs对叶片生长的影响
对转基因植株和WT的叶片进行观察,发现L:G2和35:G2的叶片均呈墨绿色且生长紧密,且L:G2的叶片数有一定增加(图5)。此外,L:G2的叶片与其他株系相比出现生长不对称和皱化的现象,推测可能是形成层内GAs含量的变化调控了植株木质部的生长,根据叶片面积发生的明显变化判断当特异性降低形成层GAs的含量时延缓了叶脉的正常发育(图5)。
2.5 下调烟草形成层GAs对不定根的影响
与对照组相比,不定根的数量在不同程度上均有所减少(图6A),通过t检验,发现其在0.01水平上差异显著,说明GAs含量的升高或降低对不定根的发育均有显著影响[9]。L:G2株系和35:G2株系不定根的生根性状在不定根数量和长度方面均相似(图6A、6B),表明特异性降低形成层中GAs的含量与降低植株整体GAs的含量对植株不定根的发育影响程度几乎相同,即木质部形成层中的活性GAs的含量是影响不定根发育的主要因素之一。
![]() 图 6 内源调控GAs含量对植株根生长发育的影响A、B为野生型和转基因植物不定根的平均数量和平均长度;C ~ F为不同转基因型植株根部,标尺长度为1 cm。C为WT株系;D为35:G2株系;E为35:G20株系;F为L:G2株系。A, B, the number and length of average adventitious roots of wild-type and transgenic plants. C−F, roots of different transgenic plants, the length of the scale is 1 cm. C, WT strain; D, 35: G2 strain; E, 35: G20 strain; F, L: G2 strain.Figure 6. Effects of endogenous regulation of GAs content on root growth and development of plants
图 6 内源调控GAs含量对植株根生长发育的影响A、B为野生型和转基因植物不定根的平均数量和平均长度;C ~ F为不同转基因型植株根部,标尺长度为1 cm。C为WT株系;D为35:G2株系;E为35:G20株系;F为L:G2株系。A, B, the number and length of average adventitious roots of wild-type and transgenic plants. C−F, roots of different transgenic plants, the length of the scale is 1 cm. C, WT strain; D, 35: G2 strain; E, 35: G20 strain; F, L: G2 strain.Figure 6. Effects of endogenous regulation of GAs content on root growth and development of plants在植株的侧根生长性状方面(图6C ~ 6F),L:G2株系几乎没有侧根产生,而35:G2的侧根与野生型WT相比有明显的增多,进一步说明特异性下调形成层内活性GAs含量对植株生长发育具有一定的调控作用,会抑制侧根的发生。
3. 讨 论
已有研究表明,赤霉素对茎部形成层、根尖的生长[9]及抗低温胁迫都有调控作用,可促进植物的生长[11-12],然而相关研究皆是以施加外源GA3或上调GAs含量为实验依据,以全株组成型调控赤霉素含量进行分析,未考虑活性赤霉素在活体内的运输特性对研究结果的影响。本研究通过对转基因及野生型烟草进行大量的观察与分析,发现特异性下调形成层赤霉素含量对植物的生长会有一定影响,如叶片不能正常伸展,木质部发育延缓,侧根和不定根的发生也会受到抑制。说明特异性调控植物局部的GAs含量对植株整体的生长发育造成了影响,且特异下调形成层区域的GAs可以达到下调植株整体GAs对木质部发育影响的效果,表明GAs在形成层中发挥了关键调控作用。
此外,利用烟草微嫁接技术将两种不同基因型的烟草嫁接后依然能生根并进行营养生长,且接穗与砧木的生长表型分别与其自身表型相同,说明活性内源GAs并不能在二者之间自由运输;而将WT//WT嫁接苗在添加有GA4+7的培养基中培养,发现嫁接苗的接穗与砧木都表现为GAs含量上调植株的表型,说明了外源的GAs通过根部吸收后,又可在体内自由运输。从以上两种结果可以判断,内源GAs和外源GAs在植物体内的运输途径可能不同,外源施加的活性GAS可能并不是通过主动运输而是通过导管组织随矿物质进行运输,而内源激素是通过形成层进行运输,这就是为什么微嫁接后内源活性GAs无法在植物体内自由运输的原因,具体机制有待于进一步的深入解析。本研究系统地证实了特异性调控形成层GAs含量在植株生长发育调控中的重要作用,为茎部赤霉素含量对植物生长发育的特异性调控机制的研究提供了理论基础,特别是赤霉素对木质部分化的特异性调控作用,为其在林木育种中的实际应用提供了依据。
-
图 1 不同产地黄栌幼苗叶功能性状对持续干旱胁迫的响应
CK. 对照;MS. 中度胁迫;SS. 重度胁迫。采用LSD检验,在α = 0.05水平上执行单因素方差分析,误差棒代表标准差。大写字母表示同一产地不同水分 处理之间的差异显著性,小写字母则表示同一水分处理时产地之间的差异显著性。CK, control; MS, moderate stress; SS, severe stress. One-way ANOVA was performed with Fisher LSD-test at α = 0.05 level. The error bars represent error of means. The capital letters indicate differences between water treatments in one location and the lowercase letters indicate differences among locations under the same treatment.
Figure 1. Responses of leaf functional traits of C. coggygria seedlings from different locations to continuous drought stress
图 2 产地分布与地理−气候因子的关系
Lat. 纬度Latitude;Lng. 经度Longitude;ANP. 年平均降水量Average annual precipitation;GSP. 生长季平均降水量Growing season precipitation;WMP. 最湿润月份降水量Precipitation of the wettest month;DMP. 最干燥月份降水量Precipitation of the driest month;GSPD. 生长季月降水量平均差Growing season mean monthly precipitation difference;RH. 平均相对湿度Mean relative humidity;ANT. 年平均气温Average annual temperature;GST. 生长季平均气温Mean temperature in growing season;GSTD. 生长季平均气温日较差 Mean daily temperature difference in growing season;WMT. 最热月份的最高气温Max. temperature of the warmest month;CMT. 最冷月份的最低气温Min. temperature of the coldest month
Figure 2. Relationship between location distribution and geographical-climatic factors
表 1 黄栌5产地地理位置及气候特征
Table 1 Geographic locations and climatic characteristics of the five C. coggygria seedling collection counties
产地
Location产地
编号
Location
code纬度
Latitude经度
Longitude年平均
降水量
Average
annual
precipitation/
mm生长季平均
降水量
Mean precipitation
in growing
season/mm最湿润月份
降水量
Precipitation of the wettest month/mm最干燥月份
降水量
Precipitation of the driest month/mm生长季月降水
量平均差
Mean monthly precipitation difference in growing season/mm北京延庆区
Yanqing District in BeijingL1 40°21′00″N 116°01′12″E 435 427.6 122.5 2.0 19.2 河北石家庄赞皇县
Zanhuang County of Shijiazhuang City in Hebei ProvinceL2 37°39′00″N 114°22′48″E 508.9 497.6 123.2 2.6 28.8 山东泰安高新区
Gaoxin District of Tai’an City in Shandong ProvinceL3 30°04′12″N 117°10′12″E 686.5 664 205.9 5.3 37.6 山西运城绛县
Jiang County of Yuncheng City in Shanxi ProvinceL4 35°28′48″N 111°34′12″E 573.5 550.5 108.6 6.3 28.3 河南三门峡陕州区
Shanzhou District of Sanmenxia City in Henan ProvinceL5 34°43′12″N 111°06′00″E 549.6 532.1 107.2 4.8 27.0 产地
Location产地
编号
Location
code平均相
对湿度
Mean relative
humidity/%年平均气温
Average annual temperature/℃生长季平
均气温
Mean temperature in growing season/℃生长季平均
气温日较差
Mean daily temperature difference in growing season/℃最热月份的
最高气温
Max. temperature of the warmest month/℃最冷月份的
最低气温
Min. temperature of the coldest month/℃北京延庆区
Yanqing District in BeijingL1 55 9.7 14.7 11.9 39.2 − 26.2 河北石家庄赞皇县
Zanhuang County of Shijiazhuang City in Hebei ProvinceL2 60 13.6 18.2 11.1 43.4 − 16.2 山东泰安高新区
Gaoxin District of Tai’an City in Shandong ProvinceL3 66 13.3 17.7 11.2 42.1 − 20.7 山西运城绛县
Jiang County of Yuncheng City in Shanxi ProvinceL4 61 11.9 16.3 10.2 39.4 − 20.5 河南三门峡陕州区
Shanzhou District of Sanmenxia City in Henan ProvinceL5 61 14.4 18.6 10.3 41.4 − 12.8 表 2 干旱胁迫、产地及其交互作用对黄栌幼苗叶功能性状影响的方差分析
Table 2 ANOVA of the influences of drought stress and location on the leaf functional traits of C. coggygria seedlings
变异来源 Source of variation df 叶绿素含量
Leaf chlorophyll content (LChl)叶相对含水量
Leaf relative water content (RWC)叶干物质含量
Leaf dry matter content (LDMC)F P F P F P 干旱胁迫 Drought stress 2 32.43 0.000 26.10 0.000 30.53 0.000 产地 Location 4 22.64 0.000 5.83 0.001 1.82 0.152 干旱胁迫 × 产地
Drought stress × location8 1.01 0.449 5.34 0.003 1.35 0.258 变异来源 Source of variation df 叶组织密度
Leaf tissue density (LD)比叶面积
Specific leaf area (SLA)叶面积比例
Leaf area ratio (LAR)F P F P F P 干旱胁迫 Drought stress 2 3.79 0.034 7.01 0.002 7.98 0.000 产地 Location 4 2.62 0.055 3.01 0.025 5.17 0.001 干旱胁迫 × 产地
Drought stress × location8 1.22 0.346 2.47 0.022 3.04 0.006 表 3 水分充足环境和干旱胁迫环境中黄栌幼苗叶功能性状之间的Pearson相关系数
Table 3 Pearson’s correlation coefficients among leaf functional traits of C. coggygria in well-irrigated and drought stress environments
叶功能性状 Leaf functional trait 叶绿素含量 LChl 叶相对含水量 RWC 叶干物质含量 LDMC 叶组织密度 LD 比叶面积 SLA 对照处理 CK 叶相对含水量 RWC 0.353 叶干物质含量 LDMC − 0.037 − 0.102 叶组织密度 LD − 0.357 − 0.312 0.286 比叶面积 SLA 0.140 0.017 − 0.080 − 0.227 叶面积比例 LAR 0.185 0.220 − 0.111 − 0.019 0.194 干旱胁迫 Drought stress 叶相对含水量 RWC 0.316** 叶干物质含量 LDMC − 0.424** − 0.455** 叶组织密度 LD − 0.481** − 0.321** 0.467** 比叶面积 SLA 0.102 0.337** − 0.398** − 0.325** 叶面积比例 LAR 0.102 0.280* − 0.320** − 0.330** 0.371** 注:**表示P < 0.01,*表示P < 0.05。Notes: ** and * represent P < 0.01 and P < 0.05, respectively. 表 4 RDA筛选出的地理−气候因子对叶片功能性状变异程度解释的贡献率(从大到小)
Table 4 Contribution of the explanation of the selected geographical-climatic factors in RDA analysis to leaf functional trait variation degree (from high to low)
地理−气候因子 Geographical-climatic factors 贡献率 Contribution/% F P 最干燥月份降水量 Precipitation of the driest month (DMP) 41.3 19.8 0.002 生长季月降水量平均差 Growing season mean monthly precipitation difference (GSPD) 38.7 12.1 0.008 最热月份的最高气温 Max. temperature of the warmest month (WMT) 17.4 6.3 0.016 年平均降水量 Average annual precipitation (ANP) 2.6 2.1 0.036 -
[1] 余华, 钟全林, 黄云波, 等. 不同种源刨花楠林下幼苗叶功能性状与地理环境的关系[J]. 应用生态学报, 2018, 29(2):449−458. Yu H, Zhong Q L, Huang Y B, et al. Relationships between leaf functional traits of Machilus pauhoi understory seedlings from different provenances and geographical environmental factors[J]. Chinese Journal of Applied Ecology, 2018, 29(2): 449−458.
[2] 张慧文, 马剑英, 孙伟, 等. 不同海拔天山云杉叶功能性状及其与土壤因子的关系[J]. 生态学报, 2010, 30(21):5747−5758. Zhang H W, Ma J Y, Sun W, et al. Altitudinal variation in functional traits of Picea schrenkiana var. tianschanica and their relationship to soil factors in Tianshan Mountains, Northwest China[J]. Acta Ecologica Sinica, 2010, 30(21): 5747−5758.
[3] 陈莹婷, 许振柱. 植物叶经济谱的研究进展[J]. 植物生态学报, 2014, 38(10):1135−1153. Chen Y T, Xu Z Z. Review on research of leaf economics spectrum[J]. Chinese Journal of Plant Ecology, 2014, 38(10): 1135−1153.
[4] 王玉平, 陶建平, 刘晋仙, 等. 不同光环境下6种常绿阔叶林树种苗期的叶片功能性状[J]. 林业科学, 2012, 48(11):23−29. doi: 10.11707/j.1001-7488.20121104 Wang Y P, Tao J P, Liu J X, et al. Response of leaf functional traits to different light regimes in an evergreen broad-leaved forest in the Jinyun Mountain[J]. Scientia Silvae Sinicae, 2012, 48(11): 23−29. doi: 10.11707/j.1001-7488.20121104
[5] 孟婷婷, 倪健, 王国宏. 植物功能性状与环境和生态系统功能[J]. 植物生态学报, 2007, 31(1):150−165. doi: 10.3321/j.issn:1005-264X.2007.01.019 Meng T T, Ni J, Wang G H. Plant functional traits, environments and ecosystem functioning[J]. Chinese Journal of Plant Ecology, 2007, 31(1): 150−165. doi: 10.3321/j.issn:1005-264X.2007.01.019
[6] 李颖, 姚婧, 杨松, 等. 东灵山主要树种在不同环境梯度下的叶功能性状研究[J]. 北京林业大学学报, 2014, 36(1):72−77. Li Y, Yao J, Yang S, et al. Leaf functional traits of main tree species at different environmental gradients in Dongling Mountain, Beijing[J]. Journal of Beijing Forestry University, 2014, 36(1): 72−77.
[7] Males J, Griffiths H. Functional types in the Bromeliaceae: relationships with drought-resistance traits and bioclimatic distributions[J]. Functional Ecology, 2017, 31: 1868−1880. doi: 10.1111/1365-2435.12900
[8] Sánchez-Gómez D, Zavala M A, Valladares F. Functional traits and plasticity linked to seedlings’ performance under shade and drought in Mediterranean woody species[J]. Annuals of Forest Science, 2008, 65(3): 311. doi: 10.1051/forest:2008004
[9] Donovan L A, Maherali H, Caruso C M, et al. The evolution of the worldwide leaf economics spectrum[J]. Trends in Ecology & Evolution (Personal edition), 2011, 26(2): 88−95.
[10] 冯秋红, 史作民, 董莉莉. 植物功能性状对环境的响应及其应用[J]. 林业科学, 2008, 44(4):125−131. doi: 10.3321/j.issn:1001-7488.2008.04.023 Feng Q H, Shi Z M, Dong L L. Response of plant functional traits to environment and its application[J]. Scientia Silvae Sinicae, 2008, 44(4): 125−131. doi: 10.3321/j.issn:1001-7488.2008.04.023
[11] 陈书文, 李娟娟, 雷新彦, 等. 观赏植物黄栌快繁技术研究[J]. 西北农林科技大学学报(自然科学版), 2005, 33(9):117−120. Chen S W, Li J J, Lei X Y, et al. Study on rapid propagateion technic for ornamental of Cotinus coggygria[J]. Journal of Northwest A&F University (Natural Science Edition), 2005, 33(9): 117−120.
[12] 孙鹏, 李金航, 刘海轩, 等. 黄栌根系结构与个体健康程度的关系[J]. 西北林学院学报, 2016, 31(2):20−27. doi: 10.3969/j.issn.1001-7461.2016.02.04 Sun P, Li J H, Liu H X, et al. Relationship between root structure and health level of Cotinus coggygria trees[J]. Journal of Northwest Forestry University, 2016, 31(2): 20−27. doi: 10.3969/j.issn.1001-7461.2016.02.04
[13] Deng Z J, Hu X F, Ai X R, et al. Dormancy release of Cotinus coggygria, seeds under a pre-cold moist stratification: an endogenous abscisic acid/gibberellic acid and comparative proteomic analysis[J]. New Forests, 2016, 47(1): 105−118. doi: 10.1007/s11056-015-9496-2
[14] 陆秀君, 董胜君, 毛红玉. 黄栌容器育苗及其对苗木耐旱性的影响[J]. 北京林业大学学报, 2001, 23(增刊):30−31. Lu X J, Dong S J, Mao H Y. Study on container seedling-raising of Cotinus coggygria var. pubescens and its effect on seedling’s drought resistance[J]. Journal of Beijing Forestry University, 2001, 23(Suppl.): 30−31.
[15] 李红云, 李焕平, 杨吉华, 等. 4种灌木林地土壤物理性状及抗侵蚀性能的研究[J]. 水土保持学报, 2006, 20(3):13−16. doi: 10.3321/j.issn:1009-2242.2006.03.004 Li H Y, Li H P, Yang J H, et al. Study on soil physical properties and anti-erosion capability under four kinds of shrubbery[J]. Journal of Soil and Water Conservation, 2006, 20(3): 13−16. doi: 10.3321/j.issn:1009-2242.2006.03.004
[16] 李金航, 齐秀慧, 徐程扬, 等. 华北4产地黄栌幼苗根系形态对干旱胁迫的短期响应[J]. 北京林业大学学报, 2014, 36(1):48−54. Li J H, Qi X H, Xu C Y, et al. Short term responses of root morphology to drought stress of Cotinus coggygria seedlings from four varied locations in northern China[J]. Journal of Beijing Forestry University, 2014, 36(1): 48−54.
[17] 李金航, 齐秀慧, 徐程扬, 等. 黄栌幼苗叶片气体交换对干旱胁迫的短期响应[J]. 林业科学, 2015, 51(1):29−41. Li J H, Qi X H, Xu C Y, et al. Short-term responses of leaf gas exchange characteristics to drought stress of Cotinus coggygria seedlings[J]. Scientia Silvae Sinicae, 2015, 51(1): 29−41.
[18] 杨晓霞, 冷平生, 郑健, 等. 暴马丁香不同种源种子和幼苗的表型性状变异及其与地理−气候因子的相关性[J]. 植物资源与环境学报, 2016, 25(3):80−89. doi: 10.3969/j.issn.1674-7895.2016.03.10 Yang X X, Leng P S, Zheng J, et al. Variation of phenotypic traits of seed and seedling of Syringa reticulata subsp. amurensis from different provenances and their correlations with geographic-climatic factors[J]. Journal of Plant Resources and Environment, 2016, 25(3): 80−89. doi: 10.3969/j.issn.1674-7895.2016.03.10
[19] 安海龙, 谢乾瑾, 刘超, 等. 水分胁迫和种源对黄柳叶功能性状的影响[J]. 林业科学, 2015, 51(10):75−84. An H L, Xie Q J, Liu C, et al. Effects of water stress and provenance on leaf functional traits of Salix gordejevii[J]. Scientia Silvae Sinicae, 2015, 51(10): 75−84.
[20] 白雪卡, 刘超, 纪若璇, 等. 种源地气候对蒙古莸光响应特性的影响[J]. 生态学报, 2018, 38(23):8425−8433. Bai X K, Liu C, Ji R X, et al. Effects of origin climate on light response characteristics of Caryopteris mongholica[J]. Acta Ecologica Sinica, 2018, 38(23): 8425−8433.
[21] Ramírez-Valiente J A, Koehler K, Cavenderbares J. Climatic origins predict variation in photoprotective leaf pigments in response to drought and low temperatures in live oaks (Quercus series Virentes)[J]. Tree Physiology, 2015, 35(5): 521−534. doi: 10.1093/treephys/tpv032
[22] 李永华, 卢琦, 吴波, 等. 干旱区叶片形态特征与植物响应和适应的关系[J]. 植物生态学报, 2012, 36(1):88−98. Li Y H, Lu Q, Wu B, et al. A review of leaf morphology plasticity linked to plant response and adaption characteristics in arid ecosystems[J]. China Journal of Plant Ecology, 2012, 36(1): 88−98.
[23] 靳泽辉, 苗峻峰, 张永端, 等. 华北地区极端降水变化特征及多模式模拟评估[J]. 气象科技, 2017, 45(1):91−100. Jin Z H, Miao J F, Zhang Y D, et al. Characteristics of extreme precipitation and its multi-model simulation evaluation in North China[J]. Meteorological Science and Technology, 2017, 45(1): 91−100.
[24] 刘大川, 周磊, 武建军. 干旱对华北地区植被变化的影响[J]. 北京师范大学学报(自然科学版), 2017, 53(2):222−228. Liu D C, Zhou L, Wu J J. Drought impacts on vegetation changes in North China[J]. Journal of Beijing Normal University (Natural Science), 2017, 53(2): 222−228.
[25] 王涛, 罗艳, 钟亦鸣, 等. 西北与华北地区现代降水变化趋势的对比[J]. 水文, 2017, 45(1):91−100. Wang T, Luo Y, Zhong Y M, et al. Comparison of recent precipitation tendency between Northwest and North China[J]. Journal of China Hydrology, 2017, 45(1): 91−100.
[26] 李岚, 王厚领, 赵琳, 等. 异源表达Peu-miR473a增强拟南芥的抗旱性[J]. 北京林业大学学报, 2015, 37(5):30−39. Li L, Wang H L, Zhao L, et al. Heterogeneous expression of Peu-miR473a gene confers drought tolerance in Arabidopsis thaliana[J]. Journal of Beijing Forestry University, 2015, 37(5): 30−39.
[27] 朱济友, 于强, 刘亚培, 等. 植物功能性状及其叶经济谱对城市热环境的响应[J]. 北京林业大学学报, 2018, 40(9):72−81. Zhu J Y, Yu Q, Liu Y P, et al. Response of plant functional traits and leaf economics spectrum to urban thermal environment[J]. Journal of Beijing Forestry University, 2018, 40(9): 72−81.
[28] Maseda P H, Fernández R J. Growth potential limits drought morphological plasticity in seedlings from six Eucalyptus provenances[J]. Tree Physiology, 2016, 36(2): 243.
[29] Valladares F, Sanchez-Gomez D, Zavala M A. Quantitative estimation of phenotypic plasticity: bridging the gap between the evolutionary concept and its ecological applications[J]. Journal of Ecology, 2006, 94(6): 1103−1116. doi: 10.1111/j.1365-2745.2006.01176.x
[30] 朱济友, 于强, Di Y, et al. 叶生态特征及其相关性对下垫面热效应的生态权衡[J]. 农业机械学报, 2018, 49(1):201−209. Zhu J Y, Yu Q, Di Y., et al. Ecological balance of leaf ecological characteristics and their correlation to thermal effects of underlying surfaces[J]. Transactions of The Chinese Society of Agricultural Machinery, 2018, 49(1): 201−209.
[31] Gholami M, Rahemi M, Rastegar S. Use of rapid screening methods for detecting drought tolerant cultivars of fig (Ficus carica L.)[J]. Scientia Horticulturae, 2012, 143: 7−14. doi: 10.1016/j.scienta.2012.05.012
[32] Chaves M M, Maroco J P, Pereira J S. Understanding plant responses to drought - from genes to the whole plant[J]. Functional Plant Biology, 2003, 30(3): 239−264. doi: 10.1071/FP02076
[33] Marron N, Dreyer E. Impact of successive drought and re-watering cycles on growth and specific leaf area of two Populus × canadensis (Moench) clones, ‘Dorskamp’ and ‘Lusisa_Avanzo’[J]. Tree Physiology, 2003, 23(18): 1225−1235. doi: 10.1093/treephys/23.18.1225
[34] Anderegg L D L, Hillerislambers J. Drought stress limits the geographic ranges of two tree species via different physiological mechanisms[J]. Global Change Biology, 2016, 22(3): 1029−1045. doi: 10.1111/gcb.13148
[35] Reich P B. The world-wide ‘fast-slow’ plant economics spectrum: a traits manifesto[J]. Journal of Ecology, 2014, 102(2): 275−301. doi: 10.1111/1365-2745.12211
[36] Volaire F. Plant traits and functional types to characterise drought survival of pluri-specific perennial herbaceous swards in Mediterranean areas[J]. European Journal of Agronomy, 2008, 29(2−3): 116−124. doi: 10.1016/j.eja.2008.04.008
-
期刊类型引用(7)
1. 王为,雷俊杰,简佶沛,王利宝. 基于云平台智能灌溉控制系统的油茶苗水分管理研究. 现代农业科技. 2023(20): 110-113+121 .  百度学术
百度学术
2. 董诗芬,王自洪,李丽华,李看清. 不同覆盖措施对初植腾冲红花油茶生长的影响研究. 林业调查规划. 2022(03): 118-121+142 .  百度学术
百度学术
3. 谢胤,余祖华,尹必期,王自洪,寸明辉,徐志映,吴兴波,杨忠品. 腾冲红花油茶主要营养器官含水率年内变化分析. 林业与环境科学. 2021(01): 25-28 .  百度学术
百度学术
4. 胡玉玲,蔡芳丽,卢海燕,罗海秀,贺姣凤. 油茶林地夏季水分管理对油茶产量指标的影响. 江苏林业科技. 2018(03): 23-27+45 .  百度学术
百度学术
5. 刘嘉翔,赵丹,杨建伟,史宝胜. 不同土壤水分条件下北京山梅花生长与耗水特性研究. 河北农业大学学报. 2018(05): 84-89 .  百度学术
百度学术
6. 何小三,徐林初,龚春,王玉娟,刘新亮,赵攀,左继林,俞元春. 干旱胁迫对‘赣无12’苗期光合特性的影响. 中南林业科技大学学报. 2018(12): 52-61 .  百度学术
百度学术
7. 樊星火,樊文勇,黄辉,施重阳,郑永红. 夏季不同灌溉方式对油茶叶片生理指标和花期的影响. 林业科技通讯. 2017(12): 12-14 .  百度学术
百度学术
其他类型引用(4)




 下载:
下载: