Adaptability response of root architecture of Cotinus coggygria seedlings to soil nutrient stress
-
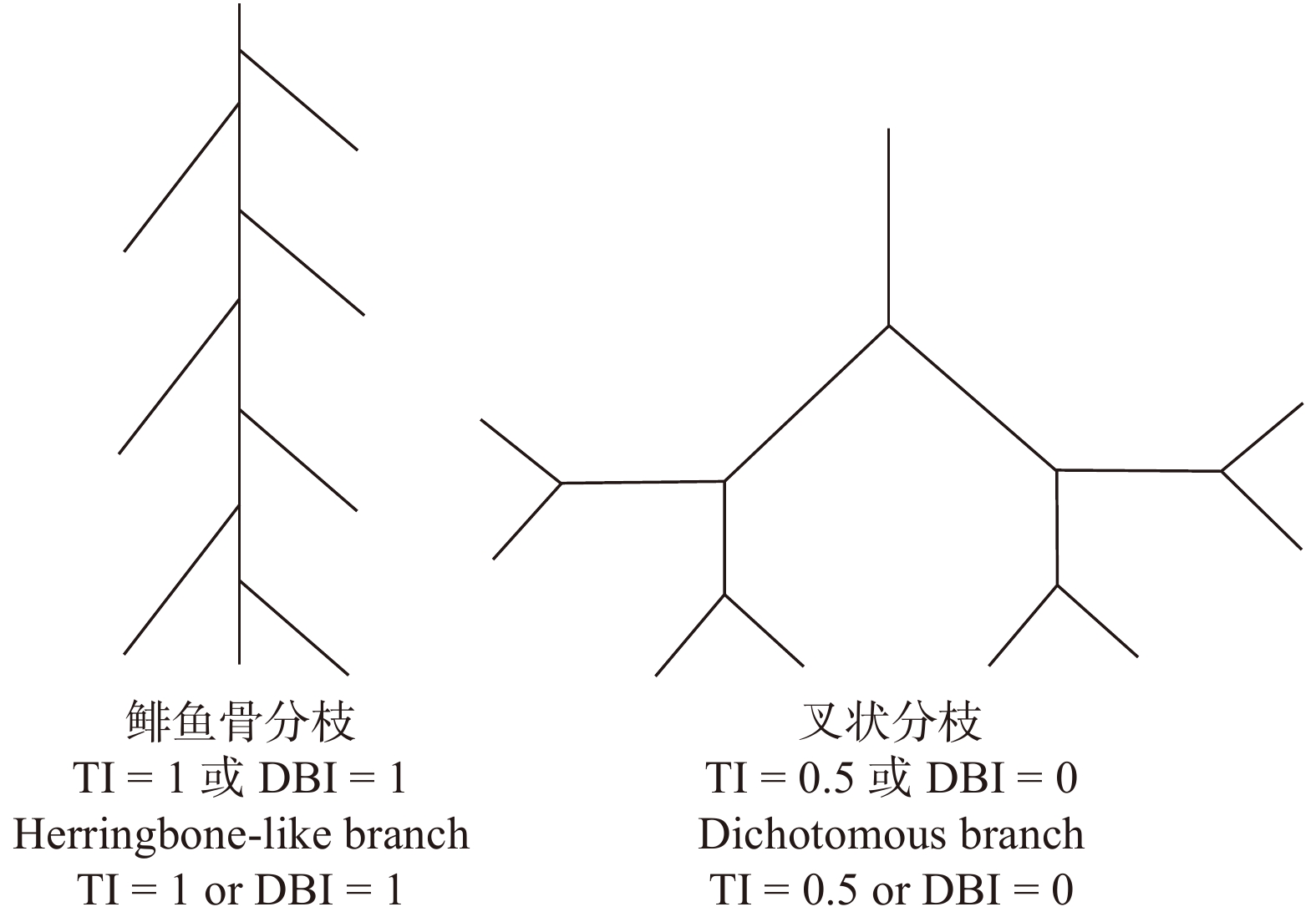
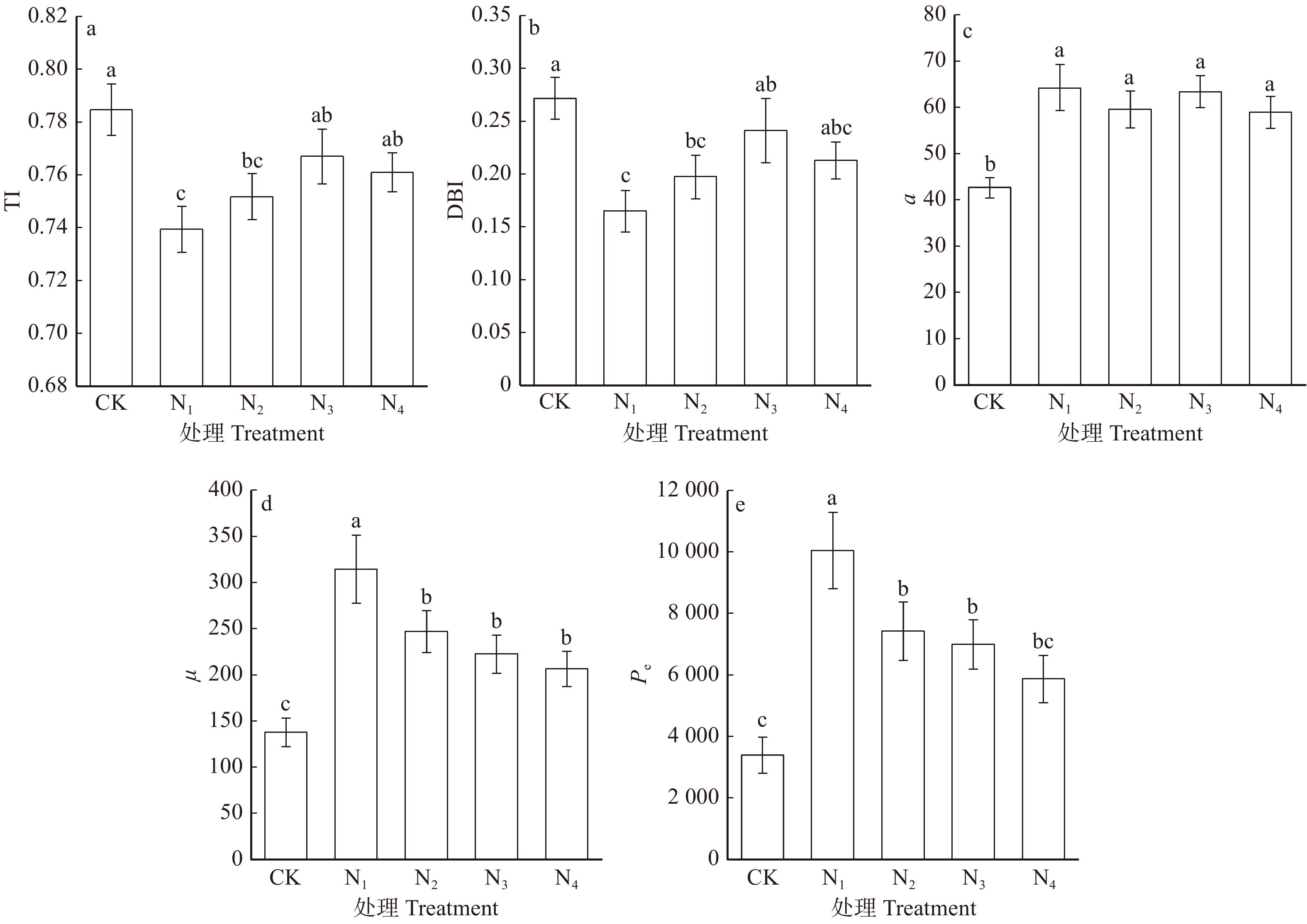
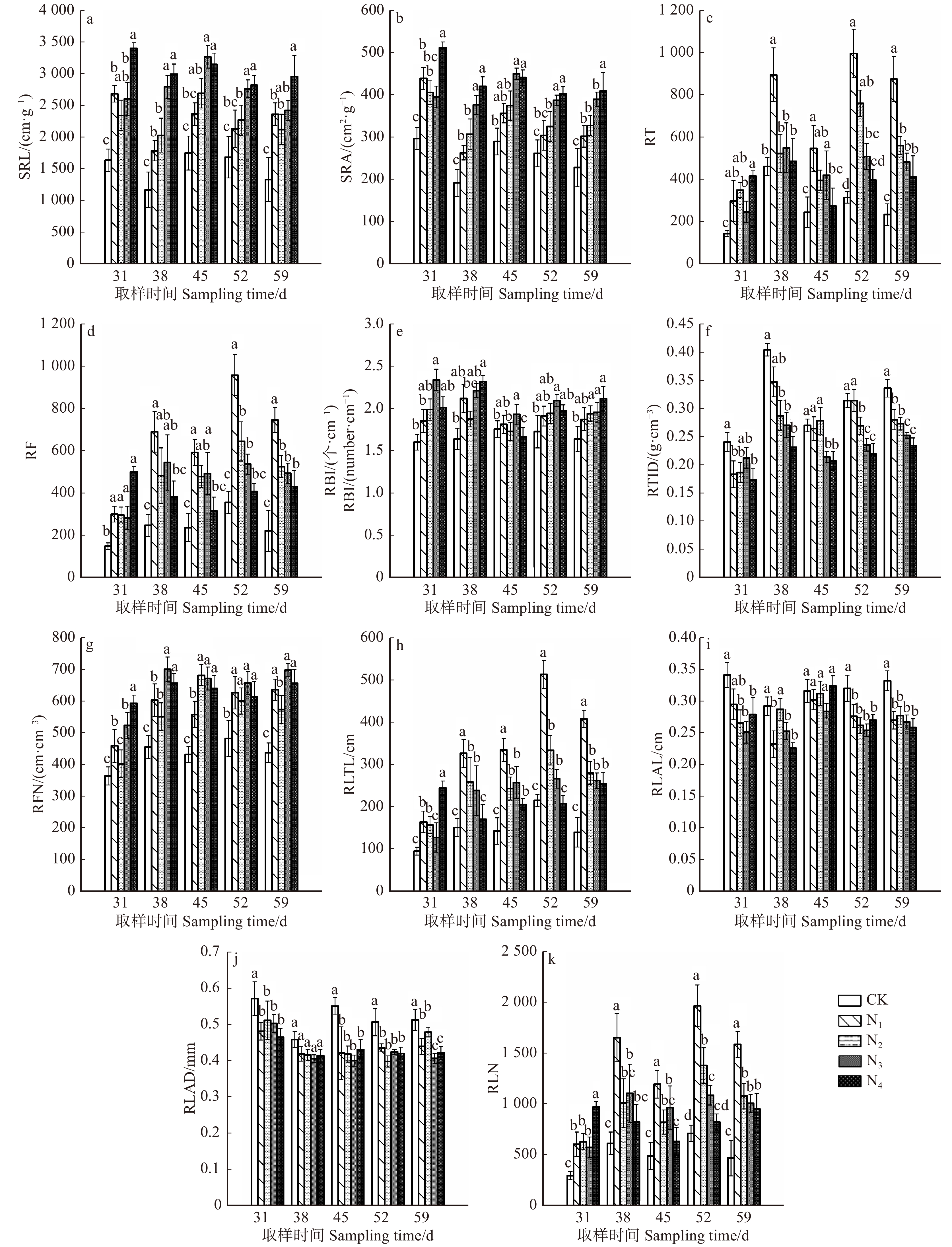
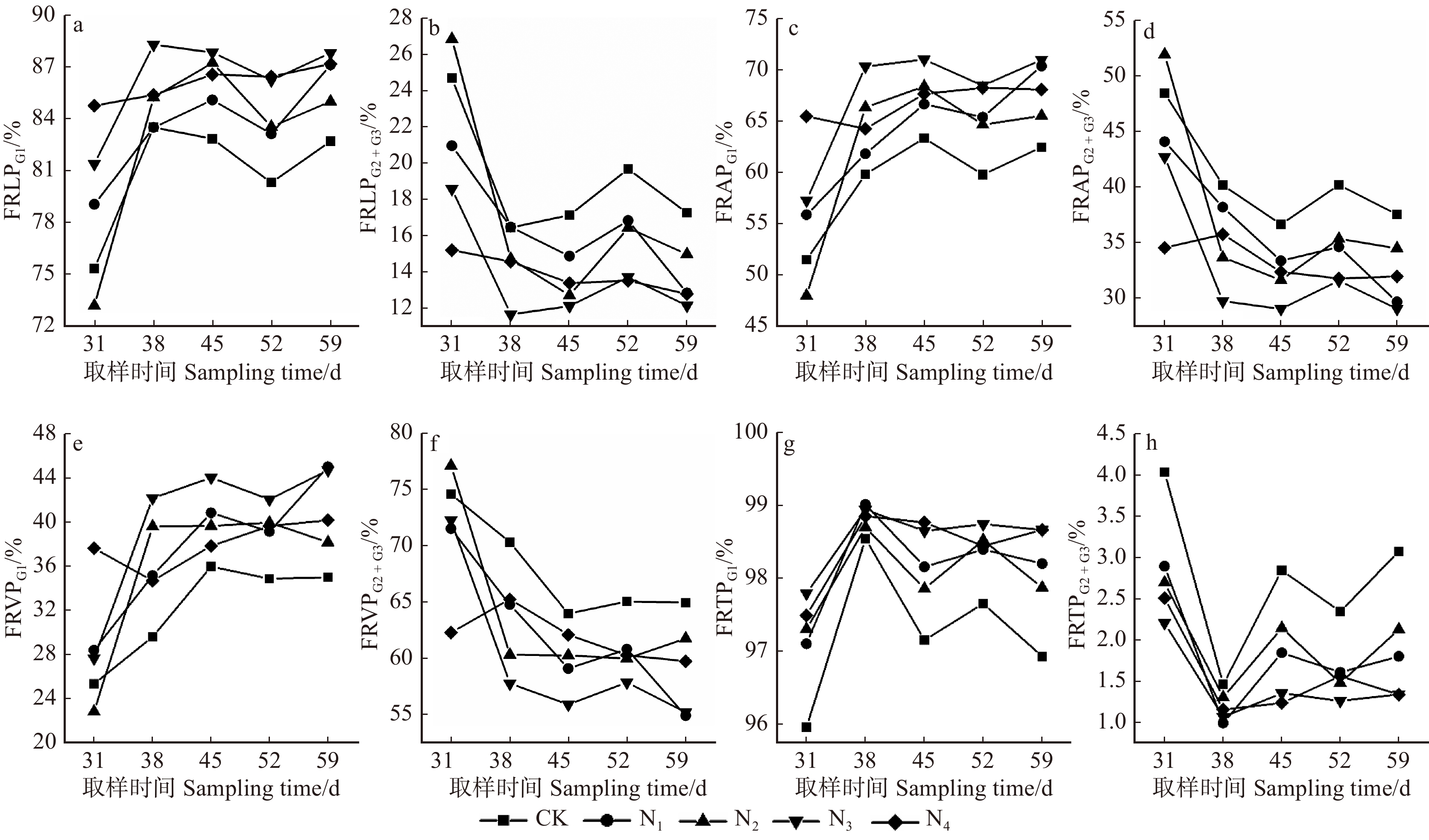
摘要:目的通过研究黄栌幼苗根系构型对土壤养分胁迫环境的适应性,探讨黄栌根系构型在土壤养分供应不足环境中的适应对策。方法试验材料为一年生黄栌幼苗,采用盆栽模拟控制试验,设置养分充足(即全土,土壤含沙比例0,CK),轻度养分胁迫(土壤含沙比例30%,N1),中度养分胁迫(土壤含沙比例50%,N2),重度养分胁迫(土壤含沙比例70%,N3)和极度养分胁迫(即全沙,土壤含沙比例100%,N4)5个梯度,分别在移栽后第31、38、45、52和59天取样,研究幼苗根系分枝模式、几何特征和不同径级细根形态在上述养分环境中的变化规律。结果(1)CK处理幼苗的根系拓扑指数(TI和DBI)最大,最长链接路径的链接数(a)、根系外部链接数(μ)以及所有链接路径的链接总数(Pe)最小。N1环境中,幼苗的TI和DBI最小,a、μ和Pe最大,分别比CK高53.2%、131.6%和194.7%。(2)N1、N2、N3和N4处理中:幼苗比根长(SRL)、比表面积(SRA)和分枝密度(RBI)逐渐增大,其中,N4处理幼苗的SRL、SRA和RBI分别比CK高67.7% ~ 157.4%、52.3% ~ 120.7%和14.7% ~ 42.1%;根尖数量(RT)、根链接数量(RLN)、根链接总长度(RLTL)和分枝数量(RF)逐渐减小,以N1处理为最大,分别比CK高95.0% ~ 279.6%、104.3% ~ 247.4%、77.4% ~ 193.5%和102.6% ~ 235.0%;根链接平均长度(RLAL)、根链接平均直径(RLAD)和根组织密度(RTID)均呈减小趋势,以N4处理最小,分别比CK低15.2% ~ 22.7%、9.3% ~ 21.4%和32.4% ~ 42.7%。(3)CK环境中,SRL和SRA的正相关系数最大(0.951),N4环境中,RF与RLN之间和RLAL和RLAD之间的正相关系数最大(分别为0.989、0.904)。N1、N2和N3处理的RBI和RLAL之间的负相关系数分别为− 0.915、− 0.889和− 0.893。(4)N3和N4处理中,0 ~ 0.50 mm范围内细根的平均长度比例、平均表面积比例、平均体积比例和平均根尖数量比例均高于其他处理,分别达86.3%和86.1%、67.6%和66.7%、40.2%和38.1%、98.6%和98.4%,而上述指标在CK处理中均为最低,分别为80.9%、59.4%、32.2%和97.2%。结论(1)土壤养分充足时,黄栌根系最接近分枝少且结构相对简单的鲱鱼骨分枝模式,具有向土壤深处延伸生长的倾向。轻度胁迫环境中,黄栌构建了分枝多、次级根重叠度高的叉状分枝模式,主、侧根尽可能地主动向距离根基较远的土壤中拓展以吸收更大范围内的养分。中度、重度和极度胁迫环境中,幼苗为减少碳消耗而采取相对简单化的根系结构对策,根系通过形成短而细的密集横向分枝(以细根为主)而加强原位利用养分能力。(2)改变根系构型几何特征参数间的协同或权衡关系、实现自身资源利用的经济化,也是黄栌应对不同土壤养分环境的重要方面。(3)从全土到极端严重胁迫环境,0 ~ 0.50 mm范围细根的分化明显增强。重度胁迫逆境时,0 ~ 0.50 mm的细根是幼苗吸收养分的重要活跃位点。极度胁迫逆境时,黄栌通过促进产生一定数量的寿命相对较长、周转速率相对较慢的细根(0.50 ~ 2.00 mm)以减少细根周转对碳的消耗,从而维持根系资源利用效率。Abstract:ObjectiveThe study was conducted to explore the adaptability strategy of root architecture of Cotinus coggygria seedlings to soil nutrient stress environment.MethodTaking one-year-old C. coggygria seedlings as research object and carrying out pot-culturing simulation control test, five soil nutrient gradients were set: nutrient-rich condition (sand proportion 0, CK), slight nutrient stress condition (sand proportion 30%, N1), medium nutrient stress condition (sand proportion 50%, N2), severe nutrient stress condition (sand proportion 70%, N3) and extreme nutrient stress condition (sand proportion 100%, N4). Seedlings were sampled at 31th, 38th, 45th, 52th and 59th day after transplanting to explore the changing rules of branching pattern and geometric characteristics of seedling root, fine root morphology at varied diameter classes under the above nutrient conditions.Result(1) Topological indices (TI or DBI) under CK were higher than the other treatments, but the number of root longest link path (a), the number of root external link (μ) and the total number of all root link paths (Pe) were lowest. Seedlings in the N1 treatment showed the lowest TI and DBI while the highest a, μ and Pe , which were higher than those in CK by 53.2%, 131.6% and 194.7%, respectively. (2) In the N1, N2, N3 and N4 treatments, specific root length (SRL), specific root surface area (SRA) and root branching index (RBI) increased gradually with the nutrient stress degree rising. Among which, SRL, SRA and SRA of seedlings under N4 treatment were higher than those in CK by 67.7%−157.4%, 52.3%−120.7% and 14.7%−42.1%, respectively.The number of root tip (RT), the number of root link (RLN), root link total length (RLTL) and the branching number of root (RF) decreased with the nutrient stress degree rising. All these four parameters in N1 treatment were highest, and higher than those in CK by 95.0%−279.6%, 104.3%−247.4%, 77.4%−193.5% and 102.6%−235.0%, respectively. Root link average length (RLAL), root link average diameter (RLAD) and root tissue density (RTID) had a reducing trend with the nutrient stress degree rising, and RLAL, RLAD and RTID in N4 were lower than those in CK by 15.2%−22.7%, 9.3%−21.4% and 32.4%−42.7%, respectively. (3) The positive correlation coefficient (0.951) between SRL and SRA was highest under CK environment, and the positive correlation coefficients between RF and RLN (0.989) and between RLAL and RLAD (0.904) were highest under N4 environment. The negative correlation coefficients under N1, N2 and N3 treatments were − 0.915, − 0.889 and − 0.893, respectively. (4) The average length proportion, average surface area proportion, average volume proportion and the average tip number proportion of fine roots in 0−0.50 mm were all higher than the other treatments, and they were 86.3% and 86.1%, 67.6% and 66.7%, 40.2% and 38.1%, 98.6% and 98.4% in N3 and N4, respectively. While these parameters under CK were lowest, which were 80.9%, 59.4%, 32.2% and 97.2%, respectively.Conclusion(1) The root system of C. coggygria in soil nutrient-rich environment is closest to the herringbone pattern with few branches and a relatively simple structure. It also has an extending tendency towards deeper layers of soil. In contrast, C. coggygria develops increased branches and a high degree of secondary root overlap in slight nutrient stress soil. Under medium, severe and extreme nutrient stress conditions, the relatively simple root structure strategy is adopted, and the root system strengthens the ability of local nutrient utilization by forming short, thin and dense lateral branches (mainly fine roots). (2) The adjustment of coordination and trade-off relationships between geometric characteristic parameters of root architecture for economizing soil resource utilization is also an important aspect for C. coggygria to cope with different soil nutrient environments. (3) The differentiation of fine roots in 0−0.50 mm is markedly enhanced from soil nutrient-rich to extreme nutrient stress environment. Fine roots in 0−0.50 mm diameter are the significantly active organs for C. coggygria to absorb nutrients in severe stress environment. When the soil is extremely poor, a certain number of fine roots of 0.50−2.00 mm diameter are promoted production to reduce the carbon consumption caused by fine root turnover, and then to maintain the root resource utilization efficiency.
-
Keywords:
- Cotinus coggygria /
- root architecture /
- soil nutrient stress /
- adaptive strategy
-
赤霉素(GAs)是一种重要植物激素,在植物整个生命周期中对生长发育的各个阶段具有广泛的调控作用[1-2]。GAs通过促进细胞分裂和细胞伸长[3-6]进而促进茎部节间的伸长生长。李哲馨[7]的研究表明GAs在烟草茎部形成层发挥重要作用,对植株生长和木质部发育具有明显的促进作用。因此,GAs代谢调控在速生林林木育种中具有重要的应用潜力;然而,以往的研究都是基于施加外源活性GAs或全株组成型调控GAs含量的分析,并没有考虑活性GAs在活体内的运输特性对研究结果的影响,有研究表明移动的GAs对茎的次生生长与木质部形成层的发育具有直接的促进作用[8]。另外,由于GAs参与调控多个发育过程,特异下调茎部GAs含量在延缓茎部生长的同时,也会抑制不定根的发生[9],影响根尖的径向生长[10]。有研究表明茎部GAs在低温胁迫的响应过程中具有反馈调控作用[11-12],因此,开展对茎部特异性GAs含量的调控研究,为GAs代谢调控植物生长发育在林木育种中的实际应用提供理论基础。
形成层位于木质部和韧皮部之间,是一种重要的分生组织,细胞分裂旺盛,向外分裂形成新的韧皮部细胞,向茎的中轴方向分裂形成新的木质部细胞,大量调控木材合成的基因在此过程中表达[13]。研究发现在形成层细胞分裂时,GAs加快了形成层细胞的分裂速度,促进了细胞的伸长,从而促进导管及筛管的伸长[14]。此后的研究中进一步证实了形成层GAs对植株生长发育有促进作用,但在外源施加GAs时,尚不能排除由于植物组织损伤而造成植株自身激素含量变化的误差,并且植物某一发育时间或某一组织中的外源激素含量的测定也难以精准,给实验带来很多不确定性[15]。因此,特异性降低植物形成层GAs含量对植物生长发育的调控作用有待于进一步深入研究。
已有研究表明,在植物体内自由运输的可能并不是活性GAs,而是其前体GA12[16]。植物嫁接是研究活性物质长距离运输特性的有效手段[17]。本实验通过嫁接不同激素含量的转基因植株及野生型WT植株再配合外施赤霉素进行实验分析,验证活性GAs在烟草中的运输机制,更深入地探讨组织特异性GAs含量对植株生长发育的调控作用,对今后GAs调控植物各组织发育的研究具有重要意义,为林木速生优质新品种定向培育策略的制定提供理论基础。
1. 材料与方法
1.1 实验材料
以本实验室遗传转化获得的烟草转基因株系LMX5:: PtGA2ox1、35S:: PtGA20ox、35S:: PtGA2ox1及野生型烟草为研究材料(其中35S:: PtGA20ox、35S:: PtGA2ox1来自林木遗传育种实验室的保存材料),分别记作L:G2、35:G20、35:G2与WT。PtGA2ox1基因克隆于毛白杨,转基因受体为野生型烟草W38株系(Nicotiana tabacum cultivar Wisconsin 38)。所有植物材料均在不含任何激素的MS培养基上,组培室温度为25 ℃,光照周期为16 h光照/8 h黑暗,将继代培养2月苗龄的生根苗移栽到温室,在30 ℃自然光照下培养。
1.2 烟草形成层赤霉素特异性调控转基因株系的获得
用毛白杨的成熟叶片提取高质量RNA,反转录获得cDNA,再以cDNA为模板克隆赤霉素代谢中的关键基因PtGA2ox1,构建表达载体LMX5::PtGA2ox1,并转化表达载体到农杆菌中。通过农杆菌介导的转基因技术获得以木质部形成层特异性启动子(LMX5)启动的烟草转基因株系L:G2[18]。
1.3 基因表达分析及GAs含量的测定
分别提取转基因烟草L:G2、35:G2及WT植株茎部、叶片和根部组织的总RNA,反转录得到cDNA,根据差异片段序列设计引物,半定量RT-PCR反应条件:95 ℃变性3 min,随后进行以下40个循环:95 ℃变性3 s,60 ℃退火20 s,72 ℃延伸30 s。以Actin为内标基因,扩增引物序列为FW:5′-CACAAGCCAGCACTTCAACAG-3′,RV:5′-ATGCCTTAACCAGGAGGTGC-3′。将每个扩增反应做3次重复实验,每次均设置阴性对照,并利用软件Bio-Rad CFXManager 2.0读取相关数据。
分别取组织培养30 d的35:G20、35:G2、L:G2与WT植株的叶、茎、根3个部位的样品各3 g,将其放在研钵中进行研磨,用LC-MS(液相色谱−质谱联用仪)对样品进行测定分析[19],每个样本进行3次重复实验。
1.4 烟草的微嫁接
以35:G2烟草转基因株系的顶芽作接穗、35:G20植株茎杆的一段作砧木,从砧木的横截面中心向下垂直的劈开切接口,使劈口长度与接穗的削面相吻合,再用锡箔纸将接穗与砧木紧密包裹,得到以35:G2为接穗,35:G20为砧木的独立嫁接苗(以下写作为35:G2//35:G20),以同样方法分别获得嫁接苗35:G20//35:G2、35:G2//WT以及WT//WT。
1.5 植株各部分生长性状的测定
挑选培养45 d的无菌生根苗L:G2、35:G2、35:G20与WT,分别对其茎部进行切片,用0.5%番红染液染色后对木质部进行显微观察并拍照。
将烟草转基因株系L:G2、35:G2和35:G20的顶芽扦插在无激素的MS培养基上,设置WT为对照,培养20 d后对各组的生根情况进行观察,再从4组株系中各选取30株,对其不定根的长度进行测量,计算平均值后与对照组一同进行t检验。
2. 结果与分析
2.1 转基因株系的获得及基因表达分析
通过农杆菌介导的转基因技术获得了以木质部形成层特异性启动子LMX5介导的烟草转基因株系L:G2,提取植株的DNA进行PCR扩增反应进行初步鉴定,成功获得21个转基因株系,与野生型WT相比,转基因植株(图1A)生长矮小,不定根数目减少,差异明显。
![]() 图 1 不同烟草植株及基因表达水平A是不同烟草植株,标尺长度为1 cm;B是转基因株系各组织中PtGA2ox1基因的表达水平;C是转基因株系不同组织中烟草NtGA2ox1基因本底表达水平,将35:G2植株中的PtGA2ox1基因的表达量设置为1。A is different tobacco plant with a scale length of 1 cm; B is the expression level of PtGA2ox1 gene in each tissue of transgenic lines; C is the background expression level of tobacco NtGA2ox1 gene in different tissues of transgenic lines, which will be in 35:G2 plants, the expression level of the PtGA2ox1 gene was set to 1.Figure 1. Different tobacco plants and gene expression levels
图 1 不同烟草植株及基因表达水平A是不同烟草植株,标尺长度为1 cm;B是转基因株系各组织中PtGA2ox1基因的表达水平;C是转基因株系不同组织中烟草NtGA2ox1基因本底表达水平,将35:G2植株中的PtGA2ox1基因的表达量设置为1。A is different tobacco plant with a scale length of 1 cm; B is the expression level of PtGA2ox1 gene in each tissue of transgenic lines; C is the background expression level of tobacco NtGA2ox1 gene in different tissues of transgenic lines, which will be in 35:G2 plants, the expression level of the PtGA2ox1 gene was set to 1.Figure 1. Different tobacco plants and gene expression levels通过测定转基因烟草L:G2、35:G2外源基因的表达水平(图1B、1C),发现L:G2、35:G2转基因植株的表达模式不同,PtGA2ox1转录水平只在L:G2的茎部出现了明显的上升趋势,而叶片和根部的上升趋势不明显。通过测定转基因烟草的叶片、根部及茎部中NtGA2ox1基因的本底表达水平,发现转基因烟草株系L:G2在叶片和根部受自身调控的NtGA2ox1基因表达水平与野生型WT对照比并无明显变化,而茎部NtGA2ox1基因的表达水平和野生型WT相比却明显降低;同时发现转基因烟草株系35:G2各组织中的NtGA2ox1基因表达水平与WT相比均有明显的下降。
在烟草中,主要活性赤霉素GA1在L:G2的茎部含量明显低于野生型WT植株,而非活性的GA8在茎部的含量与WT相比有显著的提高(图2);而转基因株系35:G20与WT相比,根、茎以及叶片内活性GAs(主要是GA1)含量显著提高,即植株整体GAs含量上调(图2);转基因植株35:G2茎、叶部分的活性GAs含量均有显著的下调,而非活性GAs含量增多,植株整体GAs含量下调,可能是GAs含量下降负反馈调节导致活性GA2-氧化酶含量下降 (图2)。
2.2 活性GAs在烟草茎中的运输特性
对35:G20//35:G2、35:G2//35:G20、及WT//WT 3组嫁接苗进行观察,发现接穗和砧木的表型性状与其各自基因型植株的性状并无较大差异(图3)。表明35:G20植株体内产生的大量活性GAs无法在嫁接植株中自由转运到35:G2中,而35:G2植株中的GA2-氧化酶也无法转化35:G20中的大量活性GAs;但培养在添加有GA4+7培养基中的WT//WT嫁接植株,相同生长时间内接穗和砧木的性状都受GAs影响,与35:G20表型相似,均表现为生长迅速,节间和叶片快速伸长,叶片颜色呈浅绿色细长的状态。
![]() 图 3 不同基因型组成的嫁接苗和不同基因型的烟草植株A.普通MS培养基中的WT//WT嫁接植株; B.添加有GA4+7的WT//WT嫁接植株; C.普通MS培养基中的35:G2//35:G20嫁接植株; D.普通MS培养基中的35:G20//35:G2嫁接植株; E.不同基因型的烟草植株。箭头代表嫁接处;A ~ D标尺长度为2 cm;E标尺长度为1 cm。The E scale is 1 cm in length; A,WT//WT grafted plants in normal MS medium; B,WT//WT grafted plants with GA4+7 were added; C, G2//35: G20 grafted plants in normal MS medium; D, 35:G20//35:G2 grafted plants in normal MS medium; E,different genotypes of tobacco plants. The arrow represents the graft; the A−D scale is 2 cm in length.Figure 3. Grafted seedlings of different genotypes and tobacco plants of different genotypes
图 3 不同基因型组成的嫁接苗和不同基因型的烟草植株A.普通MS培养基中的WT//WT嫁接植株; B.添加有GA4+7的WT//WT嫁接植株; C.普通MS培养基中的35:G2//35:G20嫁接植株; D.普通MS培养基中的35:G20//35:G2嫁接植株; E.不同基因型的烟草植株。箭头代表嫁接处;A ~ D标尺长度为2 cm;E标尺长度为1 cm。The E scale is 1 cm in length; A,WT//WT grafted plants in normal MS medium; B,WT//WT grafted plants with GA4+7 were added; C, G2//35: G20 grafted plants in normal MS medium; D, 35:G20//35:G2 grafted plants in normal MS medium; E,different genotypes of tobacco plants. The arrow represents the graft; the A−D scale is 2 cm in length.Figure 3. Grafted seedlings of different genotypes and tobacco plants of different genotypes2.3 下调烟草形成层GAs对茎部木质部生长发育的影响
通过转基因方法上调GAs含量促进植株(35:G20)生长,而下调GA含量的植株(35:G2、L:G2)生长缓慢[7],且下调植株整体GAs含量(35:G2)与特异下调形成层中的GAs含量对植株(L:G2)木质部发育的影响程度相似,说明植株GAs含量下降会使木质部的生长发育变延缓,植株的伸长生长也会受到抑制。通过茎部切片观察(图4),发现上调植株内源GAs含量(35:G20),木质部成熟提前,细胞排列整齐紧密且层数增加;下调GAs含量(35:G2、L:G2),木质部分化滞后,成熟缓慢,依据染色区域的大小发现同时期茎部相同位置的初生木质部多于WT,且细胞排列疏松不规则(图4)。转基因植株35:G2与L:G2无论是茎顶部亦或是茎基部组织中木质部的形成与发育的滞后程度、木质部细胞的排列方式等都极为相似,且下调木质部形成层的GAs含量可以达到下调植株整体GAs含量对木质部发育的影响,说明GAs影响植株木质部生长发育主要是在其形成层中发挥作用,形成层中的GAs含量对植株木质部的生长发育起关键作用。
2.4 下调烟草形成层GAs对叶片生长的影响
对转基因植株和WT的叶片进行观察,发现L:G2和35:G2的叶片均呈墨绿色且生长紧密,且L:G2的叶片数有一定增加(图5)。此外,L:G2的叶片与其他株系相比出现生长不对称和皱化的现象,推测可能是形成层内GAs含量的变化调控了植株木质部的生长,根据叶片面积发生的明显变化判断当特异性降低形成层GAs的含量时延缓了叶脉的正常发育(图5)。
2.5 下调烟草形成层GAs对不定根的影响
与对照组相比,不定根的数量在不同程度上均有所减少(图6A),通过t检验,发现其在0.01水平上差异显著,说明GAs含量的升高或降低对不定根的发育均有显著影响[9]。L:G2株系和35:G2株系不定根的生根性状在不定根数量和长度方面均相似(图6A、6B),表明特异性降低形成层中GAs的含量与降低植株整体GAs的含量对植株不定根的发育影响程度几乎相同,即木质部形成层中的活性GAs的含量是影响不定根发育的主要因素之一。
![]() 图 6 内源调控GAs含量对植株根生长发育的影响A、B为野生型和转基因植物不定根的平均数量和平均长度;C ~ F为不同转基因型植株根部,标尺长度为1 cm。C为WT株系;D为35:G2株系;E为35:G20株系;F为L:G2株系。A, B, the number and length of average adventitious roots of wild-type and transgenic plants. C−F, roots of different transgenic plants, the length of the scale is 1 cm. C, WT strain; D, 35: G2 strain; E, 35: G20 strain; F, L: G2 strain.Figure 6. Effects of endogenous regulation of GAs content on root growth and development of plants
图 6 内源调控GAs含量对植株根生长发育的影响A、B为野生型和转基因植物不定根的平均数量和平均长度;C ~ F为不同转基因型植株根部,标尺长度为1 cm。C为WT株系;D为35:G2株系;E为35:G20株系;F为L:G2株系。A, B, the number and length of average adventitious roots of wild-type and transgenic plants. C−F, roots of different transgenic plants, the length of the scale is 1 cm. C, WT strain; D, 35: G2 strain; E, 35: G20 strain; F, L: G2 strain.Figure 6. Effects of endogenous regulation of GAs content on root growth and development of plants在植株的侧根生长性状方面(图6C ~ 6F),L:G2株系几乎没有侧根产生,而35:G2的侧根与野生型WT相比有明显的增多,进一步说明特异性下调形成层内活性GAs含量对植株生长发育具有一定的调控作用,会抑制侧根的发生。
3. 讨 论
已有研究表明,赤霉素对茎部形成层、根尖的生长[9]及抗低温胁迫都有调控作用,可促进植物的生长[11-12],然而相关研究皆是以施加外源GA3或上调GAs含量为实验依据,以全株组成型调控赤霉素含量进行分析,未考虑活性赤霉素在活体内的运输特性对研究结果的影响。本研究通过对转基因及野生型烟草进行大量的观察与分析,发现特异性下调形成层赤霉素含量对植物的生长会有一定影响,如叶片不能正常伸展,木质部发育延缓,侧根和不定根的发生也会受到抑制。说明特异性调控植物局部的GAs含量对植株整体的生长发育造成了影响,且特异下调形成层区域的GAs可以达到下调植株整体GAs对木质部发育影响的效果,表明GAs在形成层中发挥了关键调控作用。
此外,利用烟草微嫁接技术将两种不同基因型的烟草嫁接后依然能生根并进行营养生长,且接穗与砧木的生长表型分别与其自身表型相同,说明活性内源GAs并不能在二者之间自由运输;而将WT//WT嫁接苗在添加有GA4+7的培养基中培养,发现嫁接苗的接穗与砧木都表现为GAs含量上调植株的表型,说明了外源的GAs通过根部吸收后,又可在体内自由运输。从以上两种结果可以判断,内源GAs和外源GAs在植物体内的运输途径可能不同,外源施加的活性GAS可能并不是通过主动运输而是通过导管组织随矿物质进行运输,而内源激素是通过形成层进行运输,这就是为什么微嫁接后内源活性GAs无法在植物体内自由运输的原因,具体机制有待于进一步的深入解析。本研究系统地证实了特异性调控形成层GAs含量在植株生长发育调控中的重要作用,为茎部赤霉素含量对植物生长发育的特异性调控机制的研究提供了理论基础,特别是赤霉素对木质部分化的特异性调控作用,为其在林木育种中的实际应用提供了依据。
-
图 4 不同养分处理中黄栌幼苗不同径级细根性状所占比例变化
FRLPG1. G1径级的细根长度比例;FRLPG2 + G3. G2 + G3径级的细根长度比例;FRAPG1. G1径级的细根表面积比例;FRAPG2 + G3. G2 + G3径级的细根表面积比例;FRVPG1. G1径级的细根体积比例;FRVPG2 + G3. G2 + G3径级的细根体积比例;FRTPG1. G1径级的细根根尖数量比例;FRAPG1. G2 + G3径级的细根根尖数量比例。FRLPG1, percentage of fine root length of G1; FRLPG2 + G3, percentage of fine root length of G2 + G3; FRAPG1, percentage of fine root surface area of G1; FRAPG2 + G3, percentage of fine root surface area of G2 + G3; FRVPG1, percentage of fine root volume of G1; FRVPG2 + G3, percentage of fine root volume of G2 + G3; FRTPG1, percentage of fine root tip number of G1; FRTPG2 + G3, percentage of fine root tip number of G2 + G3.
Figure 4. Changes of fine root trait proportions with different diameter grades of C. coggygria seedlings under different nutrient treatments
表 1 不同养分处理基质的化学性质与养分含量
Table 1 Chemical properties and nutrient contents of the tested soil under different nutrient treatments
处理
TreatmentpH 有机质
Organic matter/(g·kg− 1)总氮
Total nitrogen/(g·kg− 1)速效磷
Available phosphorus/(mg·kg− 1)速效钾
Available potassium/(mg·kg− 1)CK 8.3 7.43 0.31 14.2 321 N1 8.8 2.73 0.17 4.3 183 N2 9.0 1.88 0.11 2.2 115 N3 9.1 1.67 0.08 2.0 106 N4 9.5 1.51 0.04 0.8 94 注:CK. 养分充足;N1. 轻度养分胁迫;N2. 中度养分胁迫;N3. 重度养分胁迫;N4. 极度养分胁迫。下同。Notes:CK, nutrient-rich condition; N1, slight nutrient stress condition; N2, medium nutrient stress condition;N3, severe nutrient stress condition; N4, extreme nutrient stress condition.The same below. 表 2 养分处理对黄栌幼苗根系拓扑结构影响的方差分析
Table 2 One-way ANOVA of the influences of nutrient treatments on root topological structure of C. coggygria seedlings
根系拓扑结构参数
Root topological structure parameterdf F P a 4 6.57 < 0.001 μ 4 8.78 < 0.001 Pe 4 6.80 < 0.001 TI 4 3.89 0.005 DBI 4 3.31 0.013 注:a. 根系最长链接路径的链接数;μ.根系外部链接数;Pe.根系所有链接路径的链接数总和; TI. 拓扑指数;DBI. 叉状分枝指数。下同。Notes: a, the number of root longest link path; μ, the number of root external link; Pe, total number of all root link paths; TI, topological index; DBI, dichotomous branching index. The same below. 表 3 五个取样时期养分处理对黄栌幼苗根系构型几何特征参数影响的方差分析(P值)
Table 3 Two-way ANOVA of the influences of nutrient treatments on geometric characteristic parameters of root architecture of C. coggygria seedlings in the five sampling time (P value)
根系构型几何
特征参数
Geometric characteristic parameter of root architecture变异来源 Source of variation 取样时期
Sampling time养分处理
Nutrient stress treatment取样时期 × 养分处理
Sampling time × nutrient stress treatmentSRL 0.007 < 0.001 0.031 SRA < 0.001 < 0.001 0.036 RT < 0.001 < 0.001 < 0.001 RF < 0.001 < 0.001 0.009 RBI < 0.001 < 0.001 < 0.001 RTID < 0.001 < 0.001 0.013 RFN < 0.001 < 0.001 0.057 RLTL < 0.001 < 0.001 < 0.001 RLAL < 0.001 < 0.001 0.067 RLAD < 0.001 < 0.001 0.049 RLN < 0.001 < 0.001 0.006 注:SRL. 比根长;SRA. 比表面积;RT. 根尖数量;RF. 分枝数量;RBI. 分枝密度;RTID. 根组织密度;RFN. 根细度;RLTL. 根链接总长度;RLAL. 根链接平均长度;RLAD. 根链接平均直径;RLN. 根链接数量。下同。Notes: SRL, specific root length; SRA, specific root surface area; RT, the number of root tip; RF, the number of root branches; RBI, root branch intensity; RTID, root tissue density; RFN, root fineness; RLTL, root link total length; RLAL, root link average length; RLAD, root link average diameter; RLN, the number of root link. The same below. 表 4 不同养分处理中黄栌幼苗根系构型几何特征参数的因子载荷矩阵
Table 4 Component matrix for geometric characteristic parameters of root architecture of C. coggygria seedlings under different nutrient treatments
处理
Treatment成分
Component根系构型几何特征参数
Geometric characteristic parameters of root architecture贡献率
Contribution rate/%SRL SRA RT RF RTID RFN RLN RBI RLTL RLAL RLAD CK 1 0.427 0.218 0.879 0.896 0.242 0.751 0.939 0.103 0.947 − 0.238 − 0.479 54.1 2 0.863 0.955 − 0.122 0.148 − 0.900 0.364 0.070 0.099 0.090 − 0.034 − 0.220 21.0 3 0.172 0.144 0.236 0.281 − 0.002 0.241 0.281 0.913 0.035 − 0.944 − 0.765 14.4 N1 1 0.122 − 0.093 0.783 0.926 0.279 0.577 0.918 0.185 0.981 − 0.244 − 0.408 53.4 2 0.294 0.104 0.429 0.315 0.169 0.572 0.361 0.909 0.077 − 0.935 − 0.875 26.4 3 0.916 0.979 − 0.252 − 0.017 − 0.893 − 0.004 − 0.087 0.123 − 0.057 − 0.046 − 0.105 11.1 N2 1 0.349 0.005 0.869 0.855 0.388 0.830 0.882 0.161 0.917 − 0.202 − 0.563 50.0 2 − 0.003 0.065 0.321 0.416 − 0.220 − 0.091 0.400 0.945 0.133 − 0.945 − 0.637 24.0 3 0.924 0.981 − 0.047 − 0.036 − 0.810 0.175 − 0.040 0.048 − 0.060 − 0.100 − 0.250 14.6 N3 1 0.274 0.148 0.872 0.931 0.059 0.405 0.941 0.191 0.948 − 0.205 − 0.205 49.4 2 0.758 0.936 − 0.086 0.133 − 0.946 0.082 0.082 0.059 0.160 0.064 − 0.139 21.4 3 − 0.042 0.005 0.202 0.279 − 0.059 − 0.009 0.267 0.950 − 0.008 − 0.944 − 0.626 15.3 4 0.568 0.288 0.308 0.129 0.221 0.890 0.179 − 0.094 0.173 − 0.153 − 0.678 9.6 N4 1 0.113 0.028 0.830 0.935 − 0.058 0.113 0.941 0.436 0.967 − 0.244 − 0.223 45.5 2 0.888 0.967 − 0.122 0.111 − 0.899 0.152 0.052 − 0.003 0.149 0.069 − 0.068 24.3 3 0.057 − 0.003 0.331 0.303 0.067 0.151 0.323 0.744 0.013 − 0.938 − 0.881 15.2 4 0.425 0.194 0.288 − 0.046 0.371 0.955 0.044 − 0.432 − 0.001 − 0.097 − 0.381 11.2 表 5 不同养分处理中黄栌幼苗根系构型几何特征参数间的Pearson相关系数
Table 5 Pearson’s correlation coefficients between geometric characteristic parameters of root architecture of C. coggygria seedlings under different nutrient treatments
参数
Parameter处理
TreatmentSRA RT RF RTID RFN RLN RBI RLTL RLAL RLAD SRL CK 0.951** 0.312** 0.523** − 0.606** 0.746** 0.484** 0.268** 0.446** − 0.290* − 0.543** N1 0.921** − 0.019 0.158 − 0.666** 0.350** 0.112 0.352** 0.073 − 0.301** − 0.423** N2 0.901** 0.239* 0.238* − 0.561** 0.510** 0.245* 0.114 0.222 − 0.175 − 0.456** N3 0.924** 0.332** 0.426** − 0.545** 0.685** 0.415** 0.021 0.464** − 0.067 − 0.480** N4 0.946** 0.121 0.201 − 0.627** 0.562** 0.187 − 0.076 0.244* − 0.051 − 0.294* SRA CK 0.136 0.363** − 0.791** 0.535** 0.310** 0.241* 0.286* − 0.227 − 0.422** N1 − 0.247* − 0.078 − 0.861** − 0.023 − 0.131 0.190 − 0.143 − 0.129 − 0.151 N2 0.000 0.020 − 0.808** 0.105 0.015 0.109 − 0.018 − 0.152 − 0.241* N3 0.141 0.299** − 0.788** 0.377** 0.268* 0.062 0.338** − 0.027 − 0.350** N4 − 0.032 0.125 − 0.777** 0.314** 0.087 − 0.089 0.167 0.031 − 0.137 RT CK 0.766** 0.295** 0.647** 0.883** 0.241* 0.770** − 0.472** − 0.567** N1 0.827** 0.493** 0.622** 0.909** 0.466** 0.779** − 0.627** − 0.653** N2 0.869** 0.259* 0.596** 0.932** 0.389** 0.837** − 0.491** − 0.617** N3 0.858** 0.158 0.510** 0.918** 0.295** 0.828** − 0.448** − 0.492** N4 0.814** 0.162 0.384** 0.896** 0.477** 0.738** − 0.556** − 0.556** RF CK 0.023 0.699** 0.978** 0.453** 0.941** − 0.463** − 0.601** N1 0.298** 0.646** 0.986** 0.518** 0.948** − 0.533** − 0.625** N2 0.188 0.549** 0.989** 0.555** 0.926** − 0.503** − 0.620** N3 − 0.046 0.507** 0.991** 0.454** 0.926** − 0.455** − 0.467** N4 − 0.141 0.128 0.987** 0.646** 0.931** − 0.506** − 0.467** RTID CK − 0.003 0.112 − 0.099 0.080 − 0.002 0.024 N1 0.365** 0.367** 0.050 0.327** − 0.137 − 0.189 N2 0.340** 0.214 − 0.164 0.277* 0.160 0.014 N3 0.134 0.004 − 0.102 − 0.056 − 0.052 0.031 N4 0.209 − 0.064 − 0.118 − 0.175 − 0.144 − 0.125 RFN CK 0.721** 0.273* 0.644** − 0.381** − 0.727** N1 0.664** 0.516** 0.573** − 0.555** − 0.798** N2 0.578** 0.096 0.583** − 0.151 − 0.635** N3 0.549** 0.018 0.535** − 0.199 − 0.658** N4 0.204 − 0.222 0.133 − 0.230* − 0.532** RLN CK 0.408** 0.936** − 0.490** − 0.623** N1 0.522** 0.933** − 0.581** − 0.657** N2 0.522** 0.925** − 0.514** − 0.636** N3 0.426** 0.926** − 0.466** − 0.486** N4 0.626** 0.913** − 0.540** − 0.510** RBI CK 0.180 − 0.832** − 0.657** N1 0.266* − 0.915** − 0.820** N2 0.257* − 0.889** − 0.671** N3 0.159 − 0.893** − 0.545** N4 0.429** − 0.712** − 0.582** RLTL CK − 0.263* − 0.471** N1 − 0.318** − 0.468** N2 − 0.260* − 0.497** N3 − 0.191 − 0.371** N4 − 0.235* − 0.258* RLAL CK 0.854** N1 0.908** N2 0.807** N3 0.706** N4 0.904** 注:**和*分别代表在α = 0.01和α = 0.05水平上显著。Notes: ** and * represent significant difference at α = 0.01 and α = 0.05 level, respectively. -
[1] Fortunel C, Fine P V A, Baraloto C. Leaf, stem and root tissue strategies across 758 Neotropical tree species[J]. Functional Ecology, 2012, 26(5): 1153−1161. doi: 10.1111/j.1365-2435.2012.02020.x
[2] Ristova D, Busch W. Natural variation of root traits: from development to nutrient uptake[J]. Plant Physiology, 2014, 116(2): 518−527.
[3] 李洪波, 薛慕瑶, 林雅茹, 等. 土壤养分空间异质性与根系觅食作用: 从个体到群落[J]. 植物营养与肥料学, 2013, 19(4):995−1004. Li H B, Xue M Y, Lin Y R, et al. Spatial heterogeneity of soil nutrients and root foraging from individual to community[J]. Journal of Plant Nutrition and Fertilizer, 2013, 19(4): 995−1004.
[4] 陈伟立, 李娟, 朱红惠, 等. 根际微生物调控植物根系构型的研究进展[J]. 生态学报, 2016, 36(17):1−13. Chen W L, Li J, Zhu H H, et al. A review of the regulation of plant root system architecture by rhizosphere microorganisms[J]. Acta Ecologica Sinica, 2016, 36(17): 1−13.
[5] 郑慧玲, 赵成章, 段贝贝, 等. 琵琶柴根系分叉数与连接长度权衡关系的坡向差异[J]. 生态学杂志, 2015, 34(10):2727−2732. Zheng H L, Zhao C Z, Duan B B, et al. Differences of trade-off relationship among root forks and link length in Reaumuria soongorica on slopes of different aspects[J]. Chinese Journal of Ecology, 2015, 34(10): 2727−2732.
[6] Paez-Garcia A, Motes C M, Scheible W R, et al. Root traits and phenotyping strategies for plant improvement[J]. Plants, 2015, 4(2): 334−355. doi: 10.3390/plants4020334
[7] Rogers E D, Benfey P N. Regulation of plant root system architecture: implications for crop advancement[J]. Biotechnology, 2015, 32: 93−98.
[8] 叶子奇, 邓如军, 王雨辰, 等. 胡杨繁殖根系分枝特征及其与土壤因子的关联性[J]. 北京林业大学学报, 2018, 40(2):31−39. Ye Z Q, Deng R J, Wang Y C, et al. Branching patterns of clonal root of Populus euphratica and its associations with soil factors[J]. Journal of Beijing Forestry University, 2018, 40(2): 31−39.
[9] 单立山, 李毅, 董秋莲, 等. 红砂根系构型对干旱的生态适应[J]. 中国沙漠, 2012, 32(5):1283−1290. Shan L S, Li Y, Dong Q L, et al. Ecological adaption of Reaumuria Soongorica root system architecture to arid environment[J]. Journal of Desert Research, 2012, 32(5): 1283−1290.
[10] 李秉钧, 颜耀, 吴文景, 等. 环境因子对植物根系及其构型的影响研究进展[J]. 亚热带水土保持, 2019, 31(3):41−45. doi: 10.3969/j.issn.1002-2651.2019.03.008 Li B J, Yan Y, Wu W J, et al. Research progress on the influences of environmental factors on plant root and root architecture[J]. Subtropical Soil and Water Conservation, 2019, 31(3): 41−45. doi: 10.3969/j.issn.1002-2651.2019.03.008
[11] 杜建会, 刘安隆, 董玉祥, 等. 华南海岸典型沙生植物根系构型特征[J]. 植物生态学报, 2014, 38(8):888−895. Du J H, Liu A L, Dong Y X, et al. Architectural characteristics of roots in typical coastal psammophytes of South China[J]. Chinese Journal of Plant Ecology, 2014, 38(8): 888−895.
[12] Larson J E, Funk J L. Seedling root responses to soil moisture and the identification of a belowground trait spectrum across three growth forms[J]. New Phytologist, 2016, 210(3): 827−838. doi: 10.1111/nph.13829
[13] Fort F, Volaire F, Guilioni L, et al. Root traits are related to plant water-use among rangeland Mediterranean species[J]. Functional Ecology, 2017, 31(9): 1700−1709. doi: 10.1111/1365-2435.12888
[14] 郭京衡, 曾凡江, 李尝君, 等. 塔克拉玛干沙漠南缘三种防护林植物根系构型及其生态适应策略[J]. 植物生态学报, 2014, 38(1):36−44. doi: 10.3724/SP.J.1258.2014.00004 Guo J H, Zeng F J, Li C J, et al. Root architecture and ecological adaption strategies in three shelterbelt plant species in the southern Taklimakan Desert[J]. Chinese Journal of Plant Ecology, 2014, 38(1): 36−44. doi: 10.3724/SP.J.1258.2014.00004
[15] Fitter A H. An architectural approach to the comparative ecology of plant root systems[J]. New Phytologist, 1987, 106(1): 61−77.
[16] Fitter A H, Stickland T R, Harvey M L, et al. Architectural analysis of plant root systems (1): architectural correlates of exploitation efficiency[J]. New Phytologist, 1991, 118: 375−382. doi: 10.1111/j.1469-8137.1991.tb00018.x
[17] Fitter A H, Sticklabd T R. Architectural analysis of plant root systems (2): influence of nutrient supply on architecture in contrasting plant species[J]. New Phytologist, 1991, 118: 383−389. doi: 10.1111/j.1469-8137.1991.tb00019.x
[18] 杨小林, 张希明, 李义玲, 等. 塔克拉玛干沙漠腹地3种植物根系构型及其生境适应策略[J]. 植物生态学报, 2008, 32(6):1268−1276. doi: 10.3773/j.issn.1005-264x.2008.06.007 Yang X L, Zhang X M, Li Y L, et al. Analysis of root architecture and root adaptive strategy in the Taklimakan Desert area of China[J]. Journal of Plant Ecology, 2008, 32(6): 1268−1276. doi: 10.3773/j.issn.1005-264x.2008.06.007
[19] Beidler K V, Taylor B N, Strand A E, et al. Changes in root architecture under elevated concentrations of CO2 and nitrogen reflect alternate soil exploration strategies[J]. New Phytologist, 2015, 205(3): 1153−1163. doi: 10.1111/nph.13123
[20] Janecek S, Janecek P, Leps J. Effect of competition and soil quality on root topology of the perennial grass Molinia caerulea[J]. Preslia, 2007, 79(1): 23−32.
[21] 黄同丽, 唐丽霞, 陈龙, 等. 喀斯特区3种灌木根系构型及其生态适应策略[J]. 中国水土保持科学, 2019, 17(1):89−94. Huang T L, Tang L X, Chen L, et al. Root architecture and ecological adaption strategy of three shrubs in karst area[J]. Science of Soil and Water Conservation, 2019, 17(1): 89−94.
[22] Bouma T J, Nielsen K L, Vanhal J, et al. Root system topology and diameter distribution of species from habitats differing in inundation frequency[J]. Functional Ecology, 2001, 15(3): 360−369. doi: 10.1046/j.1365-2435.2001.00523.x
[23] 刘佳, 项文化, 徐晓, 等. 湖南会同5个亚热带树种的细根构型及功能特征分析[J]. 植物生态学报, 2010, 34(8):938−945. doi: 10.3773/j.issn.1005-264x.2010.08.006 Liu J, Xiang W H, Xu X, et al. Analysis of architecture and functions of fine roots of five subtropical tree species in Huitong, Hunan Province, China[J]. Chinese Journal of Plant Ecology, 2010, 34(8): 938−945. doi: 10.3773/j.issn.1005-264x.2010.08.006
[24] Abenavoli M R, Leone M, Sunseri F, et al. Root phenotyping for drought tolerance in bean landraces from Calabria (Italy)[J]. Journal of Agronomy and Crop Science, 2016, 202(1): 1−11. doi: 10.1111/jac.12124
[25] 张伟涛, 赵成章, 宋清华, 等. 高寒退化草地星毛委陵菜根系分叉数和连接长度的关系[J]. 生态学报, 2017, 37(24):1−7. Zhang W T, Zhao C Z, Song Q H, et al. Trade-off between root forks and link length of Potentilla acaulis in degraded alpine grassland[J]. Acta Ecologica Sinica, 2017, 37(24): 1−7.
[26] 郑慧玲, 赵成章, 徐婷, 等. 红砂根系分叉数和分支角度权衡关系的坡向差异[J]. 植物生态学报, 2015, 39(11):1062−1070. doi: 10.17521/cjpe.2015.0103 Zheng H L, Zhao C Z, Xu T, et al. Trade-off relationship between root forks and branch angle of Reaumuria songrica on different aspects of slopes[J]. Chinese Journal of Plant Ecology, 2015, 39(11): 1062−1070. doi: 10.17521/cjpe.2015.0103
[27] 宋清华, 赵成章, 史元春, 等. 不同坡向甘肃臭草根系分叉数和连接长度的权衡关系[J]. 植物生态学报, 2015, 39(6):577−585. doi: 10.17521/cjpe.2015.0055 Song Q H, Zhao C Z, Shi Y C, et al. Trade-off between root forks and link length of Melica przewalskyi on different aspects of slopes[J]. Chinese Journal of Plant Ecology, 2015, 39(6): 577−585. doi: 10.17521/cjpe.2015.0055
[28] Martinez-Sanchez J J, Ferrandis P, Trabaud L, et al. Comparative root system structure of post-fire Pinus halepensis Mill. and Cistus monspeliensis L. saplings[J]. Plant Ecology, 2003, 168(2): 309−320. doi: 10.1023/A:1024406029497
[29] 刘刚, 张光灿, 刘霞. 土壤干旱胁迫对黄栌叶片光合作用的影响[J]. 应用生态学报, 2010, 21(7):1697−1701. Liu G, Zhang G C, Liu X. Responses of Cotinus coggygria var. cinereal photosynthesis to soil drought stress[J]. Chinese Journal of Applied Ecology, 2010, 21(7): 1697−1701.
[30] 李金航, 齐秀慧, 徐程扬, 等. 黄栌幼苗叶片气体交换对干旱胁迫的短期响应[J]. 林业科学, 2015, 51(1):29−41. Li J H, Qi X H, Xu C Y, et al. Short-term responses of leaf gas exchange characteristics to drought stress of Cotinus coggygria seedlings[J]. Scientia Silvae Sinicae, 2015, 51(1): 29−41.
[31] 孙鹏, 李金航, 刘海轩, 等. 黄栌根系结构与个体健康程度的关系[J]. 西北林学院学报, 2016, 31(2):20−27. doi: 10.3969/j.issn.1001-7461.2016.02.04 Sun P, Li J H, Liu H X, et al. Relationship between root structure and health level of Cotinus coggygria trees[J]. Journal of Northwest Forestry University, 2016, 31(2): 20−27. doi: 10.3969/j.issn.1001-7461.2016.02.04
[32] 靳泽辉, 苗峻峰, 张永端, 等. 华北地区极端降水变化特征及多模式模拟评估[J]. 气象科技, 2017, 45(1):91−100. Jin Z H, Miao J F, Zhang Y D, et al. Characteristics of extreme precipitation and its multi-model simulation evaluation in North China[J]. Meteorological Science and Technology, 2017, 45(1): 91−100.
[33] 路炳军, 王志强. 北京西部山区径流小区产流产沙和土壤养分流失特征[J]. 中国水土保持科学, 2015, 13(6):33−39. doi: 10.3969/j.issn.1672-3007.2015.06.005 Lu B J, Wang Z Q. Water and nutrient losses on runoff plots in the mountainous area at western Beijing[J]. Science of Soil and Water Conservation, 2015, 13(6): 33−39. doi: 10.3969/j.issn.1672-3007.2015.06.005
[34] 史佳良, 王秀茹, 李淑芳, 等. 次降雨过程中北京市不同土地利用方式下土壤养分流失特征[J]. 水土保持学报, 2016, 30(5):58−63. Shi J L, Wang X R, Li S F, et al. Characteristics of soil nutrients loss under different land use patterns in Beijing during course of rain[J]. Journal of Soil and Water Conservation, 2016, 30(5): 58−63.
[35] 李金航, 齐秀慧, 徐程扬, 等. 华北4产地黄栌幼苗根系形态对干旱胁迫的短期响应[J]. 北京林业大学学报, 2014, 36(1):48−54. Li J H, Qi X H, Xu C Y, et al. Short term responses of root morphology to drought stress of Cotinus coggygria seedlings from four varied locations in northern China[J]. Journal of Beijing Forestry University, 2014, 36(1): 48−54.
[36] 王艺霖, 周玫, 李苹, 等. 根系形态可塑性决定黄栌幼苗在瘠薄土壤中的适应对策[J]. 北京林业大学学报, 2017, 39(6):60−67. Wang Y L, Zhou M, Li P, et al. Root morphological plasticity determining the adaptive strategies of Cotinus coggygria seedlings in barren soil environment[J]. Journal of Beijing Forestry University, 2017, 39(6): 60−67.
[37] Alvarez-Flores R, Winkel T, Nguyen-Thi-Truc A, et al. Root foraging capacity depends on root system architecture and ontogeny in seedlings of three Andean Chenopodium species[J]. Plant and Soil, 2014, 380(1−2): 415−428. doi: 10.1007/s11104-014-2105-x
[38] Birouste M, Zamora-Ledezma E, Bossard C, et al. Measurement of fine root tissue density: a comparison of three methods reveals the potential of root dry matter content[J]. Plant Soil, 2014, 374(1−2): 299−313. doi: 10.1007/s11104-013-1874-y
[39] Hodge A. Plastic plants and patchy soils[J]. Journal of Experimental Botany, 2006, 57(2): 401−411. doi: 10.1093/jxb/eri280
[40] Hodge A. The plastic plant: root responses to heterogeneous supplies of nutrients[J]. New Phytologist, 2003, 162(1): 9−24.
[41] 王庆成, 程云环. 土壤养分空间异质性与植物根系的觅食反应[J]. 应用生态学报, 2004, 15(6):1063−1068. doi: 10.3321/j.issn:1001-9332.2004.06.030 Wang Q C, Cheng Y H. Response of fine roots to soil nutrient spatial heterogeneity[J]. Chinese Journal of Applied Ecology, 2004, 15(6): 1063−1068. doi: 10.3321/j.issn:1001-9332.2004.06.030
[42] 梅莉, 王政权, 韩有志, 等. 水曲柳根系生物量、比根长和根长密度的分布格局[J]. 应用生态学报, 2006, 17(1):1−4. doi: 10.3321/j.issn:1001-9332.2006.01.001 Mei L, Wang Z Q, Han Y Z, et al. Distribution patterns of Fraxinus mandshurica root biomass, specific root length and root length density[J]. Chinese Journal of Applied Ecology, 2006, 17(1): 1−4. doi: 10.3321/j.issn:1001-9332.2006.01.001
[43] 于立忠, 丁国泉, 史建伟, 等. 施肥对日本落叶松人工林细根直径、根长和比根长的影响[J]. 应用生态学报, 2007, 18(5):957−962. doi: 10.3321/j.issn:1001-9332.2007.05.003 Yu L Z, Ding G Q, Shi J W, et al. Effects of fertilization on fine root diameter, root length and specific root length in Larix kaempferi plantation[J]. Chinese Journal of Applied Ecology, 2007, 18(5): 957−962. doi: 10.3321/j.issn:1001-9332.2007.05.003
[44] Wu Q, Pages L, Wu J. Relationships between root diameter, root length and root branching along lateral roots in adult, field-grown maize[J]. Annals of Botany, 2016, 117(3): 379−390. doi: 10.1093/aob/mcv185
[45] 何广志, 陈亚宁, 陈亚鹏, 等. 柽柳根系构型对干旱的适应策略[J]. 北京师范大学学报(自然科学版), 2016, 52(3):277−281. He G Z, Chen Y N, Chen Y P, et al. Adaptive strategy of Tamarix spp. root architecture in arid environment[J]. Journal of Beijing Normal University (Natural Sciences), 2016, 52(3): 277−281.
[46] 梅莉, 王政权, 程云环, 等. 林木细根寿命及其影响因子研究进展[J]. 植物生态学报, 2004, 28(4):704−710. Mei L, Wang Z Q, Cheng Y H, et al. A review: factors influencing fine root longevity in forest ecosystems[J]. Acta Phytoecologica Sinica, 2004, 28(4): 704−710.
[47] 张旭东, 王智威, 韩清芳, 等. 玉米早期根系构型及其生理特性对土壤水分的响应[J]. 生态学报, 2016, 36(10):1−10. Zhang X D, Wang Z W, Han Q F, et al. Effects of water stress on the root structure and physiological characteristics of early-stage maize[J]. Acta Ecologica Sinica, 2016, 36(10): 1−10.
-
期刊类型引用(7)
1. 王为,雷俊杰,简佶沛,王利宝. 基于云平台智能灌溉控制系统的油茶苗水分管理研究. 现代农业科技. 2023(20): 110-113+121 .  百度学术
百度学术
2. 董诗芬,王自洪,李丽华,李看清. 不同覆盖措施对初植腾冲红花油茶生长的影响研究. 林业调查规划. 2022(03): 118-121+142 .  百度学术
百度学术
3. 谢胤,余祖华,尹必期,王自洪,寸明辉,徐志映,吴兴波,杨忠品. 腾冲红花油茶主要营养器官含水率年内变化分析. 林业与环境科学. 2021(01): 25-28 .  百度学术
百度学术
4. 胡玉玲,蔡芳丽,卢海燕,罗海秀,贺姣凤. 油茶林地夏季水分管理对油茶产量指标的影响. 江苏林业科技. 2018(03): 23-27+45 .  百度学术
百度学术
5. 刘嘉翔,赵丹,杨建伟,史宝胜. 不同土壤水分条件下北京山梅花生长与耗水特性研究. 河北农业大学学报. 2018(05): 84-89 .  百度学术
百度学术
6. 何小三,徐林初,龚春,王玉娟,刘新亮,赵攀,左继林,俞元春. 干旱胁迫对‘赣无12’苗期光合特性的影响. 中南林业科技大学学报. 2018(12): 52-61 .  百度学术
百度学术
7. 樊星火,樊文勇,黄辉,施重阳,郑永红. 夏季不同灌溉方式对油茶叶片生理指标和花期的影响. 林业科技通讯. 2017(12): 12-14 .  百度学术
百度学术
其他类型引用(4)




 下载:
下载: