Identification and expression analysis of GST genes in response to quercetin induction in Hyphantria cunea
-
摘要:目的
谷胱甘肽S-转移酶(GSTs)是昆虫体内一种重要的解毒酶,在植食性昆虫对植物次生物质的解毒适应中发挥重要作用。本研究筛选和鉴定了美国白蛾中肠响应槲皮素诱导的GST基因,分析了槲皮素对GST基因的诱导表达模式,为阐明美国白蛾GST基因在解毒代谢槲皮素中的功能奠定基础。
方法基于槲皮素诱导的美国白蛾中肠转录组,筛选和鉴定响应槲皮素诱导的GST基因;通过构建系统发育树分析GST基因的家族类别;采用实时荧光定量PCR技术研究槲皮素对GST基因诱导的剂量效应和时间效应。
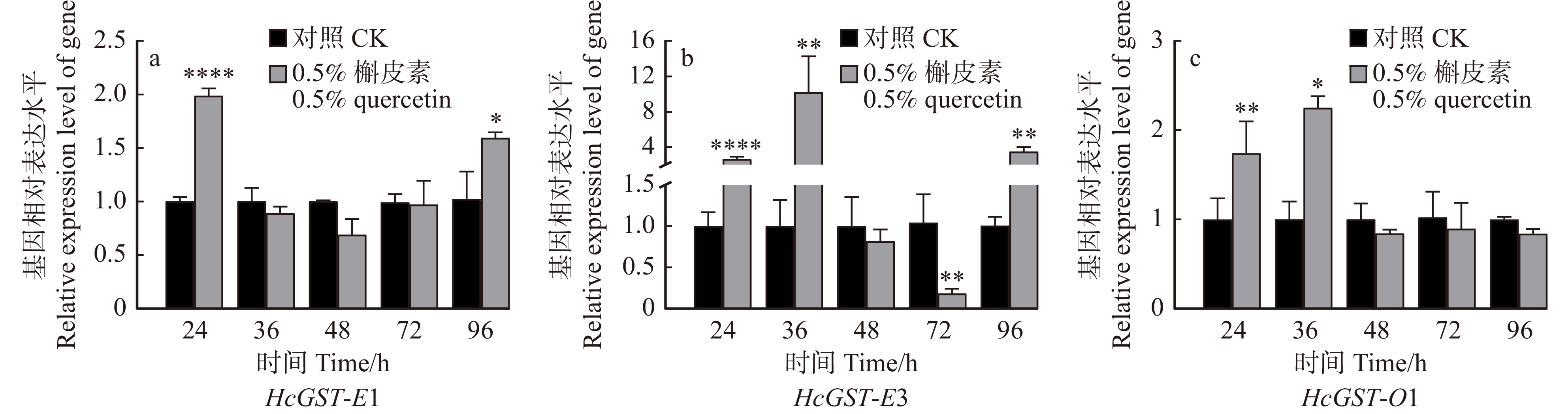
结果鉴定了美国白蛾响应槲皮素诱导的6个GST基因;系统发育分析显示HcGST-E1、HcGST-E2、HcGST-E3属于GST Epsilon家族,HcGST-S1、HcGST-S2属于GST Sigma家族,HcGST-O1属于GST Omega家族。不同质量分数槲皮素对GST基因的诱导作用不同,本实验质量分数(0.5%、1.0%、2.0%、4.0%)的槲皮素均能诱导HcGST-E1表达水平显著上调,0.5%、1.0%和 2.0%的槲皮素诱导HcGST-E3表达水平显著升高;0.5%的槲皮素诱导HcGST-O1的表达水平显著增加。本实验质量分数的槲皮素均诱导HcGST-S1显著下调表达;0.5%和1.0%的槲皮素诱导HcGST-S2的表达水平显著下调。3个上调GST基因均在24 h或36 h内显著上调表达响应槲皮素的诱导。
结论槲皮素能显著诱导美国白蛾中肠6个GST基因的表达水平,但对不同基因诱导的表达模式不同。HcGST-E1、HcGST-E3和HcGST-O1显著上调表达响应不同质量分数槲皮素的诱导,推测这3个基因是参与美国白蛾解毒代谢槲皮素的关键基因。
Abstract:ObjectiveGlutathione S-transferases (GSTs) are important detoxification enzymes in insects, playing an important role in the physiologhical adaptation of phytophagous insects to plant secondary substances. In this study, we screened and identified GST genes and analyzed their expression patterns induced by quercetin in Hyphantria cunea midgut, laying the foundation for elucidating the function of GST genes in detoxification and metabolism of quercetin in H. cunea.
MethodBased on the quercetin-induced midgut transcriptome of H. cunea, the GST genes in response to quercetin induction were screened and identified. The family categories of GST genes were analyzed by constructing phylogenetic tree. The dose effect and time effect of quercetin on GST gene induction were studied by real-time fluorescence quantitative PCR.
ResultSix GST genes were identified from the gut in response to quercetin induction. Phylogenetic analysis showed that HcGST-E1, HcGST-E2 and HcGST-E3 belong to the GST Epsilon family, HcGST-S1 and HcGST-S2 belong to the GST Sigma family, and HcGST-O1 belongs to the GST Omega family. Various mass fraction of quercetin had different induction effects on GST genes. The tested mass fraction of quercetin (0.5%, 1.0%, 2.0% and 4.0%) significantly up-regulated HcGST-E1 expression, below 2.0% of quercetin significantly increased HcGST-E3 expression, and 0.5% quercetin significantly enhanced HcGST-O1 expression. By contrast, the tested mass fraction of quercetin significantly induced the down-regulation of the expression level of HcGST-S1 and 0.5% and 1.0% of quercetin significantly down-regulated the expression level of HcGST-S2. Three up-regulated GST genes were significantly up-regulated within 24 h or 36 h by quercetin.
ConclusionQuercetin could significantly induce the expression levels of six GST genes in the midgut of H. cunea, but the expression patterns are various for each induced gene. HcGST-E1, HcGST-E3 and HcGST-O1 are significantly up-regulated in response to the quercetin induction. It is, therefore, speculated that these 3 genes are the key genes involved in the detoxification of quercetin in H. cunea.
-
Keywords:
- Hyphantria cunea /
- GST gene /
- quercetin /
- induction /
- transcriptional expression
-
-
图 1 美国白蛾与其他鳞翅目昆虫的GST基因系统发育树
Bm.家蚕;Ha.棉铃虫;Sf.草地贪夜蛾 ;Sl.斜纹夜蛾;Tn.粉纹夜蛾;Px.柑橘凤蝶;Se.甜菜夜蛾;Hc.美国白蛾。不同颜色代表不同GST基因家族;红色字体基因代表本研究鉴定的6条GST基因。节点处的数值为分支支持度。Bm, Bombyx mori; Ha, Helicoverpa armigera; Sf, Spodoptera frugiperda; Sl, Spodoptera litura; Tn, Trichoplusia ni; Px, Papilio xuthus; Se, Spodoptera exigua; Hc, Hyphantria cunea. Different colors represent different GST gene families. The red font genes represent the 6 GST genes identified in this study. The values at the node indicate branch support.
Figure 1. Phylogenetic tree of GST genes of Hyphantria cunea and other lepidoptera insects
图 2 不同质量分数槲皮素诱导24 h后美国白蛾5个GST基因的相对表达水平
条形柱上的*代表不同质量分数槲皮素诱导后,GST基因相对表达量与对照组差异显著,*代表P < 0.05,**代表P < 0.01,***代表P < 0.001,****代表P < 0.000 1。下同。The * on the bar represents that the relative expression level of GST gene is significantly different from the control after induction with different mass fractions of quercetin. * represents P < 0.05, ** represents P < 0.01, *** represents P < 0.001, **** represents P <0.000 1. The same below.
Figure 2. Relative expression level of five GST genes of Hyphantria cunea after 24 h induction with different mass fractions of quercetin
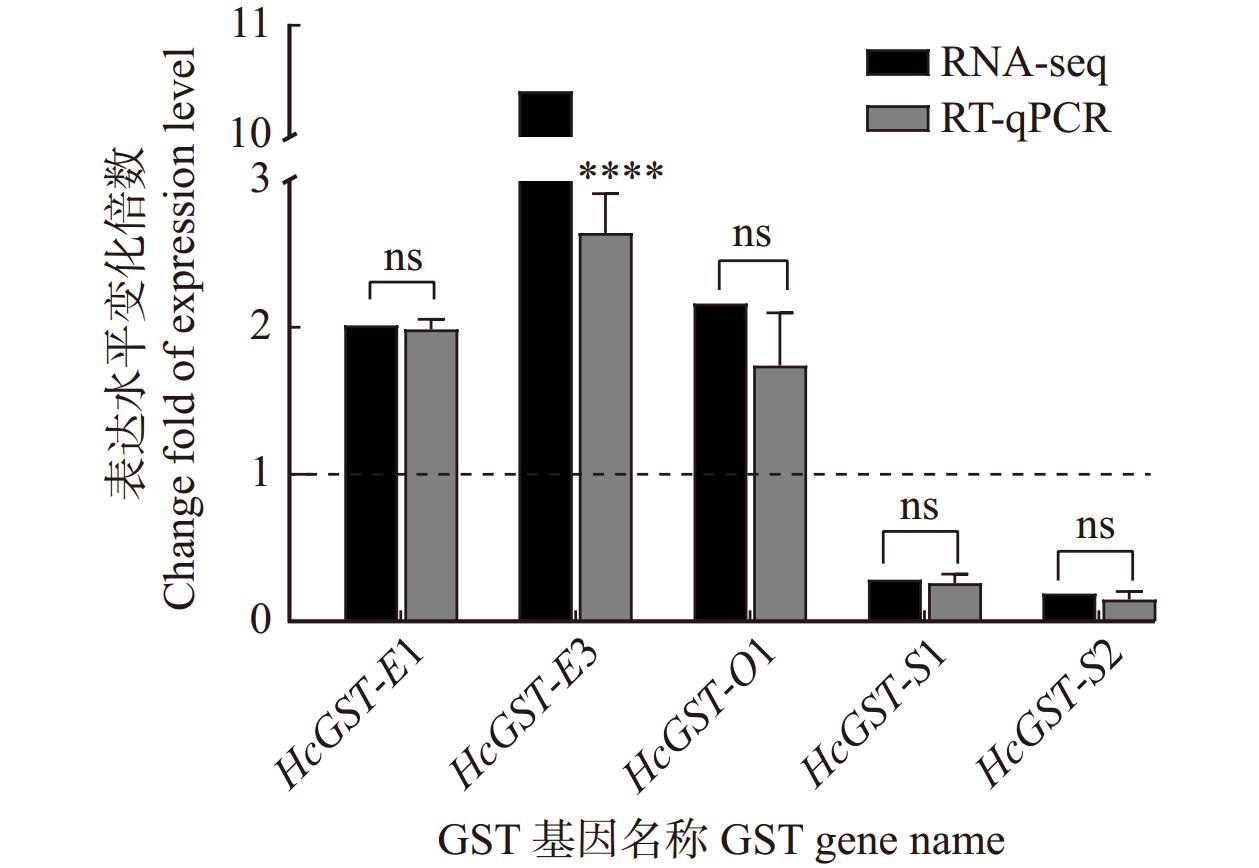
图 3 0.5%槲皮素诱导下美国白蛾中肠GST基因在转录组和RT-qPCR中的表达水平
虚线为对照组(CK)的相对表达水平。ns代表同一基因的两种表达量变化倍数差异不显著。The dotted line is the relative expression level of control group. ns represents there is no significant difference between the two expression multiples of the same gene.
Figure 3. Relative expression level in RNA-seq and RT-qPCR of GST genes in midgut of Hyphantria cunea induced by 0.5% quercetin
表 1 RT-qPCR引物序列信息
Table 1 RT-qPCR primer sequence information
基因
Gene引物序列(5′—3′)
Primer sequence (5′−3′)扩增效率
Amplification efficiency/%相关系数
Correlation coefficientHcGST-E1 F-CGAGAGCAAGACACACCTGA
R-ATAGAGACGCGTGCTTTGGT93.2 0.997 HcGST-E3 F-ACCCGCTACATACTATCCCGA
R-AACGTTGATCCACTGTCGCT110.0 0.996 HcGST-S1 F-ATGTGAGGGCTTTAGGTGAGG
R-TCAGCCCATTGTTCAGGAGTT96.2 0.997 HcGST-S2 F-CGGTAGCATGGTCGACTACTT
R-ACGGACTTTCTTGAGGGCAG93.3 0.994 HcGST-O1 F-GGTGATCCACTCCCGTCATAC
R-GGCATTAAGCGCCAAGACAG103.4 0.996 GAPDH F-GACTGGCATGGCTTTCAGAG
R-CATCGTAGCTAGCGGGTTTG96.5 0.999 EF1α F-TTATCGTCGCTGCTGGTACT
R-GAGTGTGAAAGCGAGCAGAG103.5 0.999 表 2 响应槲皮素诱导的美国白蛾GST基因的BLASTX结果
Table 2 BLASTX results of quercetin-induced GST genes in Hyphantria cunea
基因名称
Gene name登录号
Accession
No.转录水平
Transcription level编码蛋白长度
Encoded protein
length/aa同源性比较最佳匹配结果 Homology comparison of best match result 蛋白质名称
Protein name物种
SpeciesE值
E value一致性
Identity/%登录号
Accession
No.HcGST-E1 OM323338 表达上调2.02倍
Up-regulated
expression 2.02 times228 Glutathione-S-
transferase epsilon海灰翅夜蛾
Spodoptera
littoralis4.00 × e−123 73.68 AYM01156.1 HcGST-E2 OM323336 表达下调0.34倍
Down-regulated
expression 0.34 times216 Glutathione S-
transferase
epsilon 13甜菜夜蛾
Spodoptera
exigua1.00 × e−113 72.64 ASN63944.1 HcGST-E3 OM323337 表达上调10.26倍
Up-regulated
expression 10.26 times216 Glutathione S-
transferase 1烟草天蛾
Manduca sexta2.00 × e−103 65.28 XP_030020682.1 HcGST-S1 OM287126 表达下调0.29倍
Down-regulated
expression 0.29 times203 Glutathione S-
transferase 2-like棉铃虫
Helicoverpa
armigera2.00 × e−81 61.76 XP_021186026.1 HcGST-S2 OM323334 表达下调0.19倍
Down-regulated
expression 0.19 times204 Glutathione S-
transferase
sigma 4甜菜夜蛾
Spodoptera
exigua2.00 × e−93 65.84 ASN63950.1 HcGST-O1 OM323335 表达上调2.17倍
Up-regulated
expression 2.17 times284 Glutathione S-
transferase 8小线角木蠹蛾
Streltzoviella
insularis3.00 × e−116 60.34 QLI62204.1 -
[1] Treutter D. Significance of flavonoids in plant resistance: a review[J]. Environmental Chemistry Letters, 2006, 4(3): 147−157. doi: 10.1007/s10311-006-0068-8
[2] Sharma R, Sohal S K. Bioefficacy of quercetin against melon fruit fly[J]. Bulletin of Insectology, 2013, 66(1): 79−83.
[3] Li Z, Guan X, Michaud J P, et al. Quercetin interacts with Cry1Ac protein to affect larval growth and survival of Helicoverpa armigera[J]. Pest Management Science, 2016, 72(7): 1359−1365. doi: 10.1002/ps.4160
[4] Cui B, Huang X, Li S, et al. Quercetin affects the growth and development of the grasshopper Oedaleus asiaticus (Orthoptera: Acrididae)[J]. Journal of Economic Entomology, 2019, 112(3): 1175−1182. doi: 10.1093/jee/toz050
[5] 陈澄宇, 康志娇, 史雪岩, 等. 昆虫对植物次生物质的代谢适应机制及其对昆虫抗药性的意义[J]. 昆虫学报, 2015, 58(10): 1126−1139. doi: 10.16380/j.kcxb.2015.10.011 Chen C Y, Kang Z J, Shi X Y, et al. Metabolic adaptation mechanisms of insects to plant secondary metabolites and their implications for insecticide resistance of insects[J]. Acta Entomologica Sinica, 2015, 58(10): 1126−1139. doi: 10.16380/j.kcxb.2015.10.011
[6] Sheehan D, Meade G, Foley V M, et al. Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily[J]. Biochemical Journal, 2001, 360(1): 1−16. doi: 10.1042/bj3600001
[7] 张常忠, 高希武, 郑炳宗. 棉铃虫谷胱甘肽S-转移酶的活性分布和发育期变化及植物次生物质的诱导作用[J]. 农药学学报, 2001, 3(1): 30−35. doi: 10.3321/j.issn:1008-7303.2001.01.006 Zhang C Z, Gao X W, Zheng B Z. Glutathione S-transferases (GSTs) in Helicoverpa armigera: subcellular and tissue distribution of activity, developmental changes and induction of allelochemicals[J]. Chinese Journal of Applied Entomology, 2001, 3(1): 30−35. doi: 10.3321/j.issn:1008-7303.2001.01.006
[8] Hayes J D, Flanagan J U, Jowsey I R. Glutathione transferases[J]. Annual Review of Pharmacology and Toxicology, 2005, 45: 51−88. doi: 10.1146/annurev.pharmtox.45.120403.095857
[9] 高希武, 董向丽, 郑炳宗, 等. 棉铃虫的谷胱甘肽S-转移酶(GSTs): 杀虫药剂和植物次生性物质的诱导与GSTs对杀虫药剂的代谢[J]. 昆虫学报, 1997, 40(2): 122−127. doi: 10.3321/j.issn:0454-6296.1997.02.002 Gao X W, Dong X L, Zheng B Z, et al. Glutathione S-transferases (GST) of Helicoverpa armigera: induction of insecticides and plant secondary substances and metabolism of insecticides by GST[J]. Acta Entomologica Sinica, 1997, 40(2): 122−127. doi: 10.3321/j.issn:0454-6296.1997.02.002
[10] 汤方, 梁沛, 高希武. 2-十三烷酮和槲皮素诱导棉铃虫谷胱甘肽S-转移酶组织特异性表达[J]. 自然科学进展, 2005, 15(7): 805−810. doi: 10.3321/j.issn:1002-008X.2005.07.006 Tang F, Liang P, Gao X W. Tissue specific expression of glutathione S-transferase in Helicoverpa armigera induced by 2-tridecanone and quercetin[J]. Progress in Natural Science, 2005, 15(7): 805−810. doi: 10.3321/j.issn:1002-008X.2005.07.006
[11] Zhang Y E, Ma H J, Feng D D, et al. Induction of detoxification enzymes by quercetin in the silkworm[J]. Journal of Economic Entomology, 2012, 105(3): 1034−1042. doi: 10.1603/EC11287
[12] 牟少飞, 梁沛, 高希武. 槲皮素对B型烟粉虱羧酸酯酶和谷胱甘肽S-转移酶活性的影响[J]. 昆虫知识, 2006, 43(4): 491−495. Mou S F, Liang P, Gao X W. Effects of quercetin on specific activity of carboxylesterase and glutathione S-transferases in Bemisia tabaci[J]. Chinese Journal of Applied Entomology, 2006, 43(4): 491−495.
[13] 汤方, 周玉宝, 张秀波, 等. 2-十三烷酮和槲皮素对杨小舟蛾谷胱甘肽S-转移酶活性的影响[J]. 植物保护学报, 2009, 36(4): 377−378. doi: 10.13802/j.cnki.zwbhxb.2009.04.019 Tang F, Zhou Y B, Zhang X B, et al. The effect on glutathione S-transferases by 2-tridecanone and quercetin in Micromelalopha trogiodyta (Lepidoptera: Notodontidae)[J]. Acta Entomologica Sinica, 2009, 36(4): 377−378. doi: 10.13802/j.cnki.zwbhxb.2009.04.019
[14] 萧刚柔, 李镇宇. 中国森林昆虫[M]. 3版.北京: 中国林业出版社, 2020. Xiao G R, Li Z Y. Chinese forest insects[M]. 3rd ed. Beijing: China Forestry Publishing House, 2020.
[15] 杨忠岐, 张永安. 重大外来入侵害虫: 美国白蛾生物防治技术研究[J]. 昆虫知识, 2007, 44(4): 465−471. Yang Z Q, Zhang Y A. Researches on techniques for biocontrol of the fall webworm, Hyphantria cunea, a severe invasive insect pest to China[J]. Chinese Bulletin of Entomology, 2007, 44(4): 465−471.
[16] Sullivan G, Ozman-Sullivan S. Tachinid (Diptera) parasitoids of Hyphantria cunea (Lepidoptera: Arctiidae) in its native North America and in Europe and Asia: a literature review[J]. Entomologica Fennica, 2012, 23: 181−192
[17] 刘海军, 骆有庆, 温俊宝, 等. 北京地区红脂大小蠹、美国白蛾和锈色粒肩天牛风险评价[J]. 北京林业大学学报, 2005, 27(2): 81−87. Liu H J, Luo Y Q , Wen J B, et al. Pest risk assessment of Dendroctonus valens, Hyphantria cunea and Apriona swainsoni in Beijing area[J]. Journal of Beijing Forestry University, 2005, 27(2): 81−87.
[18] 潘忠玉. 3 种次生代谢物对美国白蛾幼虫生长发育及解毒酶活性的影响[D]. 北京: 北京林业大学, 2020. Pan Z Y. Effects of three secondary metabolites on the growth and development and detoxification enzyme activities in Hyphantria cunea (Lepidoptera: Arctiidae) [J]. Beijing: Beijing Forestry University, 2020.
[19] 蒋立娣, 宣贵达, 吴好好, 等. 桑叶提取物中槲皮素和山萘酚的含量测定[J]. 浙江大学学报 (理学版), 2009, 36(6): 705−707. Jiang L D, Xuan G D, Wu H H, et al. Determination of quercetin and kaempferol in folium mori extract after hydrolysis by hydrochloric acid[J]. Journal of Zhejiang University (Science Edition), 2009, 36(6): 705−707.
[20] 王海燕, 杨金龙, 谷山林, 等. 桑叶槲皮素提取物抗氧化活性研究[J]. 丝绸, 2018, 55(3): 15−20. doi: 10.3969/j.issn.1001-7003.2018.03.003 Wang H Y, Yang J L, Gu S L, et al. Study on antioxidant activity of quercetin extract from mulberry leaves[J]. Silk, 2018, 55(3): 15−20. doi: 10.3969/j.issn.1001-7003.2018.03.003
[21] 曹利军, 杨帆, 唐思莹, 等. 适合三种鳞翅目昆虫的一种人工饲料配方[J]. 应用昆虫学报, 2014, 51(5): 1376−1386. Cao L J, Yang F, Tang S Y, et al. Development of an artificial diet for three lepidopteran insects[J]. Chinese Journal of Applied Entomology, 2014, 51(5): 1376−1386.
[22] Kumar S, Stecher G, Li M, et al. MEGA X: molecular evolutionary genetics analysis across computing platforms[J]. Molecular Biology and Evolution, 2018, 35(6): 1547. doi: 10.1093/molbev/msy096
[23] Zhang D, Gao F, Jakovlić I, et al. PhyloSuite: an integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies[J]. Molecular Ecology Resources, 2020, 20: 348−55 doi: 10.1111/1755-0998.13096
[24] Nguyen L T, Schmidt H A, Haeseler A V, et al. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies[J]. Molecular Biology and Evolution, 2014, 32: 268−74.
[25] Guindon S, Dufayard J F, Lefort V, et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of phyml 3.0[J]. Systematic Biology, 2010, 59: 307−321. doi: 10.1093/sysbio/syq010
[26] Minh B Q, Nguyen M, Haeseler A V. Ultrafast approximation for phylogenetic bootstrap[J]. Molecular Biology and Evolution, 2013, 30: 1188−1195. doi: 10.1093/molbev/mst024
[27] 陶蓉, 李慧, 孙宇航, 等. 美国白蛾内参基因的鉴定及筛选[J]. 林业科学, 2019, 55(9): 111−120. Tao R, Li H, Sun Y H, et al. Indentification and screening of internal reference genes of Hyphantria cunea (Lepidoptera: Arctiidae)[J]. Scientia Silvae Sinicae, 2019, 55(9): 111−120.
[28] Livak K J, Schmittgen T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method[J]. Methods, 2001, 25: 402−408 doi: 10.1006/meth.2001.1262
[29] Enayati A A, Ranson H, Hemingway J. Insect glutathione transferases and insecticide resistance[J]. Insect Molecular Biology, 2005, 14(1): 3−8. doi: 10.1111/j.1365-2583.2004.00529.x
[30] Ranson H, Rossiter L, Ortelli F, et al. Identification of a novel class of insect glutathione S-transferases involved in resistance to DDT in the malaria vector Anopheles gambiae[J]. Biochemical Journal, 2001, 359(2): 295−304. doi: 10.1042/bj3590295
[31] Qin G, Jia M, Liu T, et al. Identification and characterisation of ten glutathione S-transferase genes from oriental migratory locust, Locusta migratoria manilensis (Meyen)[J]. Pest Management Science, 2011, 67(6): 697−704. doi: 10.1002/ps.2110
[32] Sun L, Yin J, Du H, et al. Characterisation of GST genes from the Hyphantria cunea and their response to the oxidative stress caused by the infection of Hyphantria cunea nucleopolyhedrovirus (HcNPV)[J]. Pesticide Biochemistry and Physiology, 2020, 163: 254−262. doi: 10.1016/j.pestbp.2019.11.019
[33] Feng K, Luo J, Ding X, et al. Transcriptome analysis and response of three important detoxifying enzymes to Serratia marcescens Bizio (SM1) in Hyphantria cunea (Drury) (Lepidoptera: Noctuidae)[J]. Pesticide Biochemistry and Physiology, 2021, 178: 104922. doi: 10.1016/j.pestbp.2021.104922
[34] Yamamoto K, Fujii H, Aso Y, et al. Expression and characterization of a sigma-class glutathione S-transferase of the fall webworm, Hyphantria cunea[J]. Bioscience, Biotechnology, and Biochemistry, 2007, 71(2): 553−560. doi: 10.1271/bbb.60592
[35] Yamamoto K, Zhang P B, Banno Y, et al. Identification of a sigma-class glutathione-S-transferase from the silkworm, Bombyx mori[J]. Journal of Applied Entomology, 2006, 130(9−10): 515−522.
[36] Tang F, Tu H, Shang Q, et al. Molecular cloning and characterization of five glutathione S-transferase genes and promoters from Micromelalopha troglodyta (Graeser) (Lepidoptera: Notodontidae) and their response to tannic acid stress[J]. Insects, 2020, 11(6): 339. doi: 10.3390/insects11060339
[37] 张婷, 卫正国, 高瑞娜, 等. 芸香苷诱导家蚕谷胱甘肽-S-转移酶 omega 家族基因的表达变化[J]. 蚕业科学, 2011, 37(2): 224−229. doi: 10.3969/j.issn.0257-4799.2011.02.007 Zhang T, Wei Z G, Gao R N, et al. Variation of rutin-induced expression of Glutathione-S-transferase omega family genes in Bombyx mori[J]. Science of Sericulture, 2011, 37(2): 224−229. doi: 10.3969/j.issn.0257-4799.2011.02.007
[38] 张婷, 卫正国, 高瑞娜, 等. 芸香苷对家蚕谷胱甘肽-S-转移酶部分基因的诱导表达[J]. 昆虫学报, 2011, 54(1): 20−26. doi: 10.16380/j.kcxb.2011.01.009 Zhang T, Wei Z G, Gao R N, et al. Induction of expression of partial glutathione-S-transferase genes in Bombyx mori by rutin[J]. Acta Entomologica Sinica, 2011, 54(1): 20−26. doi: 10.16380/j.kcxb.2011.01.009
[39] 马康, 邹晓鹏, 岑永杰, 等. Sli-miR-34-5p 响应植物次生物质正调控斜纹夜蛾谷胱甘肽 S-转移酶基 SlGSTe1的表达[J]. 昆虫学报, 2019, 62(1): 1−8. Ma K, Zou X P, Cen Y J, et al. Sli-miR-34-5p positively regulates the expression of the glutathione S-transferase gene SlGSTe1 in Spodoptera litura (Lepidoptera: Noctuidae) in response to secondary plant substances[J]. Acta Entomologica Sinica, 2019, 62(1): 1−8.
[40] 岑永杰, 邹晓鹏, 郑思春. miR-305-3p 和 miR-71-5p 通过调控谷胱甘肽代谢途径参与斜纹夜蛾应对植物次生物质[J]. 环境昆虫学报, 2019, 41(1): 33−41. Cen Y J, Zou X P, Zheng S C. MiR-305-3p and miR-71-5p involve in Spodoptera litura responding to phytochemical by regulating glutathione metabolism pathway[J]. Journal of Environmental Entomology, 2019, 41(1): 33−41.
[41] Ma J, Sun L, Zhao H, et al. Functional identification and characterization of GST genes in the Asian gypsy moth in response to poplar secondary metabolites[J]. Pesticide Biochemistry and Physiology, 2021, 176: 104860. doi: 10.1016/j.pestbp.2021.104860
[42] 齐琪, 孙丽丽, 许力山, 等. RNAi分析舞毒蛾谷胱甘肽S-转移酶(GST)基因对黄酮和槲皮素胁迫响应[J]. 环境昆虫学报, 2021, 43(6): 1359−1367. doi: 10.3969/j.issn.1674-0858.2021.06.03 Qi Q, Sun L L, Xu L S, et al. Response of glutathione S-transferase (GST) genes in Lymantria dispar to flavone and quercetin stresses based on RNAi analysis[J]. Journal of Environmental Entomology, 2021, 43(6): 1359−1367. doi: 10.3969/j.issn.1674-0858.2021.06.03
[43] Han J B, Li G Q, Wan P J, et al. Identification of glutathione S-transferase genes in Leptinotarsa decemlineata and their expression patterns under stress of three insecticides[J]. Pesticide Biochemistry and Physiology, 2016, 133: 26−34. doi: 10.1016/j.pestbp.2016.03.008
-
期刊类型引用(11)
1. 赵熙来,周正,葛锐,罗伟豪,马旭彤,蒋慧,苏华维. 残次香梨与乳酸菌组合对四翅滨藜青贮的影响. 中国饲料. 2024(17): 155-161 .  百度学术
百度学术
2. 张衡锋,杨绮,韦庆翠,张焕朝. 盐胁迫对10个品种紫薇的影响及其耐盐性综合评价. 东北林业大学学报. 2023(09): 34-40 .  百度学术
百度学术
3. 李雨欣,罗秀丽,张婷婷,康宇乾,王鹏,江行玉,周扬. 盐胁迫下海马齿生理指标变化及相关基因表达分析. 农业生物技术学报. 2022(07): 1279-1289 .  百度学术
百度学术
4. 刘鹤莹,张嫚,翟中葳,杨鹏,支苏丽,沈仕洲,张克强. 大薸对奶厅废水主要污染物的去除效果研究. 农业环境科学学报. 2022(11): 2525-2538 .  百度学术
百度学术
5. 王涛,蒙仲举,张佳鹏,雷虹娟,张格. NaCl胁迫对紫穗槐幼苗生长及生理特性的影响. 西北林学院学报. 2021(01): 25-30 .  百度学术
百度学术
6. 刘学良,姚俊修,刘翠兰,李善文,任飞,李庆华,吴海涛,翟红莲,吴德军,邢世岩,高红萍. 7个接骨木无性系苗木对盐胁迫的生理响应与评价. 中南林业科技大学学报. 2021(01): 37-44+79 .  百度学术
百度学术
7. 鲁俊倩,武舒,钟姗辰,张伟溪,苏晓华,张冰玉. ‘84K’杨组氨酸激酶基因PaHK3a的表达及功能分析. 北京林业大学学报. 2021(02): 46-53 .  本站查看
本站查看
8. 丁丁,王红宝,郑伶杰,左永梅,韩民利,吴新海,郭艳超. 不同品种茶菊对NaCl胁迫的生理响应及耐盐性评价. 植物生理学报. 2021(03): 692-702 .  百度学术
百度学术
9. 赵佳伟,李清亚,路斌,李艳,朱玉菲,栗浩,路丙社. 不同品种北美豆梨对NaCl胁迫的生理响应及耐盐性评价. 植物生理学报. 2019(01): 23-31 .  百度学术
百度学术
10. 邹晓君,列志旸,薛立. NaCl胁迫对4种园林植物养分含量和贮量的影响. 华南农业大学学报. 2018(06): 77-84 .  百度学术
百度学术
11. 杨传宝,孙超,李善文,姚俊修,刘敬国,矫兴杰. 白杨派无性系苗期耐盐性综合评价及筛选. 北京林业大学学报. 2017(10): 24-32 .  本站查看
本站查看
其他类型引用(5)




 下载:
下载: