Effects of NaHCO3 stress on growth, photosynthesis and chlorophyll fluorescence characteristics in Populus davidiana × P. bolleana overexpressed TaLEA
-
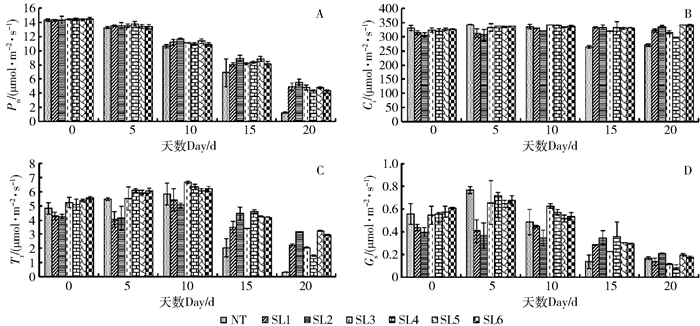
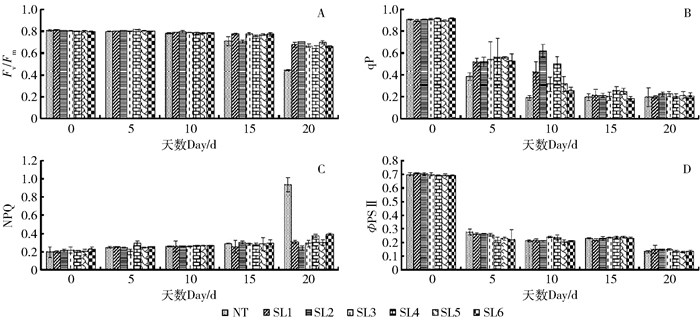
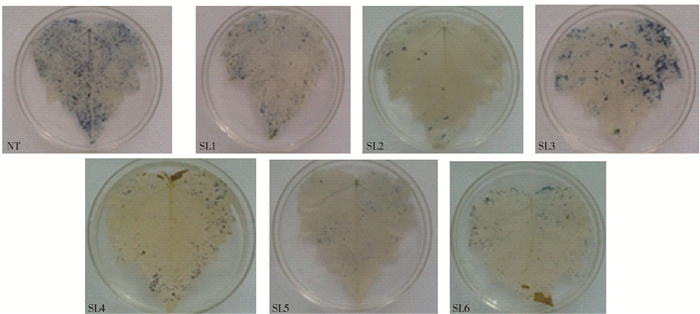
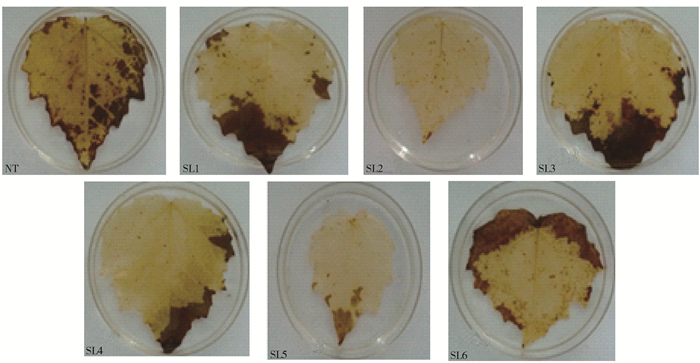
摘要: 为了筛选耐苏打盐渍土的转基因杨树优良株系, 以苏打盐渍土的主要成分NaHCO3胁迫处理转TaLEA基因的山新杨各株系与对照(NT),测定不同株系的苗高和地径等生长性状,测定净光合速率(Pn)、胞间CO2浓度(Ci)、气孔导度(Gs)、蒸腾速率(Tr)等光合参数以及最大光化学效率(Fv/Fm)、光化学猝灭系数(qP)、非光化学猝灭系数(NPQ)、实际光化学电子产量(ΦPSII)等叶绿素荧光动力学参数,利用染色法分别比较各株系的超氧离子和过氧化氢的累积情况,进而综合评价不同株系对NaHCO3胁迫的响应。结果表明,NaHCO3胁迫处理前、后各株系的苗高和地径生长量发生明显分化,由处理前的差异不显著,到处理20 d后的显著,转基因株系的苗高、地径普遍较对照高,表现突出的是转基因株系SL2号,其平均苗高和地径分别较对照提高了17.4%、15.7%;对照的盐害指数是转基因株系的3.3倍,并且转基因株系的平均存活率是对照的2.8倍。各转基因株系的光合参数和荧光参数也较对照增强,Pn、Tr在胁迫20 d后转基因株系较对照分别高了237.38%、649.02%,Gs、Ci在胁迫15 d时转基因株系较对照分别高了119.05%、24.56%,其中SL2号转基因株系较对照分别提高150%、25.81%。各转基因株系的Fv/Fm平均为0.68,对照只有0.45,前者的qP和ΦPSII只略高于后者,后者的NPQ却是前者的6倍。转基因株系的超氧离子和过氧化氢累积量均少于对照。在相同的碱胁迫环境下,转基因山新杨,尤其是SL2,能保持较快的生长量、较强的光合能力和较低的ROS水平,初步选择其为耐苏打盐渍土的杨树转基因优良株系。Abstract: In order to find the transgenic poplar which can tolerate soda-saline soil, the lines overexpressed TaLEA gene and the control (NT) were treated with NaHCO3, the main components of soda-saline soil. And to evaluate salt-tolerant properties, we measured some parameters of different lines, including height and ground diameter, net photosynthetic rate(Pn), intercellular CO2 concentration(Ci), stomatal conductance(Gs), transpiration rate(Tr), maximal photochemical efficiency(Fv/Fm), photochemical quenching coefficient (qP), non-photochemical quenching coefficient (NPQ), and the actual photochemical electron yield (ΦPSII). Moreover, the accumulation of superoxide anion and hydrogen peroxide in each line was compared by staining method. The results showed that obvious changes had taken place in height and ground diameter among different lines after NaHCO3 stress. There was no significant difference before treatment, but after treating for 20 days, significant differences happened. Compared with control, all transgenic lines increased in height and ground diameter, especially the SL2 transgenic line, whose average height and ground diameter were increased by 17.4% and 15.7%, respectively. Furthermore, the salt damage index of wild type was 3.3 times of transgenic lines, and the survival rate of transgenic lines was 2.8 times of wild type. The photosynthetic parameters and fluorescence parameters of the transgenic lines after 20 days stress were also higher than wild type, Pn and Tr were increased by 237.38% and 649.02%, respectively compared with the wild type. Compared with control, Gs and Ci of transgenic lines were increased by 119.05% and 24.56% under 15 days stress, and Gs, Ci of SL2 transgenic line were increased by 150% and 25.81%. Besides, the average Fv/Fm of each transgenic line was 0.68, the wild type was 0.45. The qP and PSII of transgenic plants were only slightly higher than that of the wild type, but the NPQ of wild type was 6 times of wild type.The accumulation of superoxide anion and hydrogen peroxide in transgenic lines was less than that in wild type. All in all, transgenic lines, especially SL2, could maintain rapid growth, strong photosynthetic capacity and lower level of ROS under alkali stress environment, so SL2 was initially selected as an excellent transgenic line of poplar because of salinity tolerance.
-
Keywords:
- transgene /
- NaHCO3 stress /
- growth /
- photosynthesis /
- chlorophyll fluorescence /
- TaLEA
-
-
图 1 碱性盐害级值
A.0级无盐害症状;B.1级轻度盐害(有部分叶尖、叶缘变黄);C.2级中度盐害(有大约1/2的叶片、叶缘焦枯);D.3级重度盐害(大部分叶片、叶缘焦枯或脱落);E.4级极重度盐害(复水后植株死亡)。
Figure 1. Level of alkaline salt stress
A, symptom of 0 salt injury; B, level 1 for mild salt damage (the tip of leaf or leaf margin turned yellow); C, level 2 for moderate salt damage (about 1/2 blade or leaf margin turned withered); D, level 3 for severe salt damage (most of the leaf, leaf margin turned withered or was off); E, level 4 for extremely severe salt damage (plant dead after rewatering).
图 3 NaCHO3胁迫对叶片叶绿素荧光动力学参数的影响
A.最大光化学效率(Fv/Fm);B.光化学猝灭系数(qP);C.非光化学猝灭系数(NPQ);D.实际光化学电子产量(ΦPSII)。
Figure 3. Effects of NaCHO3 stress on chlorophyll fluorescence kinetics parameters of leaves
A, maximum photochemical efficiency (Fv/Fm); B, photochemical quenching coefficient (qP); C, non-photochemical quenching coefficient (NPQ); D, actual photochemical electron yield (ΦPSII).
表 1 NaHCO3胁迫前后高生长比较
Table 1 Comparison in height growth before and after NaHCO3 stress
株系
Line胁迫前、后平均苗高
Average height before and after stress/cm非胁迫条件下
平均苗高
Average height
without stress/cmNaHCO3对高生长的
影响Effect of
NaHCO3 on height
growth/%胁迫前
Before stress胁迫后
After stress绝对高生长
Absolute height growthNT 85.55±3.67 122.11±5.02 c 36.55±5.40 b 147.78±4.42 17.37±2.07ab SL1 84.33±2.36 130.17±2.79 bc 46.00±8.38 ab 147.17±1.01 11.55±1.53ab SL2 88.67±3.21 143.33±7.23 a 54.44±6.16 a 149.67±8.21 4.21±1.75d SL3 85.33±2.60 128.89±6.50 bc 43.56±5.54 ab 145.00±4.58 10.96±7.31bc SL4 85.56±2.14 122.19±2.72 c 37.08±3.83 b 150.67±5.69 18.80±4.36 a SL5 86.30±4.39 132.89±6.44 b 46.92±7.87 ab 139.22±5.42 4.57±1.20 cd SL6 87.05±1.25 127.22±3.53 bc 40.17±2.42 b 143.17±2.56 11.10±3.59ab 平均Mean 86.11±2.80 129.54±4.89 43.53±5.66 146.09±4.55 11.21±2.73 注:不同小写字母表示在P < 0.05水平上差异显著。下同。Notes:different lowercase letters mean significant differences at P < 0.05 level. Same as below. 表 2 NaHCO3胁迫前后地径生长比较
Table 2 Comparison in ground diameter growth before and after NaHCO3 stress
株系
Line胁迫前、后平均地径
Average ground diameter before and after stress/mm非胁迫条件下
平均地径Mean
ground diameter
without stress/mmNaHCO3对地径生长
的影响Effect of
NaHCO3 on ground
diameter growth/%胁迫前
Before stress胁迫后
After stress绝对地径生长
Absolute ground diameter growthNT 2.18±0.017 6.48±0.133 c 4.30±0.51 7.53±0.59 13.57±7.71 SL1 2.16±0.10 7.22±0.18 ab 5.06±0.15 8.18±0.63 11.68±8.57 SL2 2.23±0.036 7.50±0.055 a 5.27±0.38 8.49±0.42 11.60±4.02 SL3 2.04±0.058 6.80±0.084 bc 4.77±0.26 7.88±0.63 13.24±7.11 SL4 2.18±0.079 6.92±0.38 bc 4.68±0.35 8.04±0.46 13.87±5.64 SL5 2.18±0.085 6.71±0.36 c 4.53±0.78 7.99±0.47 15.64±8.71 SL6 2.20±0.046 6.79±0.26 bc 4.58±0.35 7.96±0.49 14.50±6.86 平均Mean 2.17±0.063 6.92±0.23 4.74±0.40 8.01±0.52 13.44±6.94 表 3 碱性盐害指数及存活率
Table 3 Index of salt-alkaline stress and survival rate
株系
Line碱害指数
Index of salt-alkaline stress存活率
Survival rate/%NT 0.79 30 SL1 0.13 87 SL2 0.28 100 SL3 0.30 80 SL4 0.18 90 SL5 0.22 90 SL6 0.34 62 -
[1] 马驰.松嫩平原土地盐碱化研究[J].吉林农业大学学报, 2014, 36(3):333-337. http://d.old.wanfangdata.com.cn/Periodical/jlnydxxb201403015 MA C. Research on soil salinization in Songnen Plain[J]. Journal of Jilin Agricultural University, 2014, 36(3):333-337. http://d.old.wanfangdata.com.cn/Periodical/jlnydxxb201403015
[2] 张晓光, 黄标, 梁正伟, 等.松嫩平原西部土壤盐碱化特征研究[J].土壤, 2013, 45(2):1332-1338. http://d.old.wanfangdata.com.cn/Periodical/tr201302023 ZHANG X G, HUANG B, LIANG Z W, et al. Study on salinization characteristics of surface soil in western Songnen Plain[J]. Soils, 2013, 45(2):1332-1338. http://d.old.wanfangdata.com.cn/Periodical/tr201302023
[3] 刘正祥, 张华新, 杨秀艳, 等.林木耐盐碱相关基因与基因工程研究进展[J].世界林业研究, 2012, 25(5):11-17. http://d.old.wanfangdata.com.cn/Periodical/sjlyyj201205003 LIU Z X, ZHANG H X, YANG X Y, et al. Research advances in gene and genetic engineering for saline-alkaline tolerance of forest trees[J]. World Forestry Research, 2012, 25(5):11-17. http://d.old.wanfangdata.com.cn/Periodical/sjlyyj201205003
[4] ZHAO X, ZHAN L P, ZOU X Z. Improvement of cold tolerance of the half-high bush Northland blueberry by transformation with the LEA gene from Tamarix androssowii[J]. Plant Growth Regulation, 2011, 63(1):13-22. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=d3893487f7ceac0bb725e5686db22a39
[5] 师静, 刘美芹, 史军娜, 等.沙冬青胚胎晚期发生丰富蛋白基因序列及表达特性分析[J].北京林业大学学报, 2012, 34(4):114-119. http://j.bjfu.edu.cn/article/id/9786 SHI J, LIU M Q, SHI J N, et al. Sequence analysis and expression pattern of AmLEA14 encoding a late embryogenesis abundant protein in Ammopiptanthus mongolicus[J]. Journal of Beijing Forestry University, 2012, 34(4):114-119. http://j.bjfu.edu.cn/article/id/9786
[6] ZHAN J Y, KONG L F, LIU Z P, et al. Stress-induced expression Arabidopsis with a Dehydrin LEA protein from Cleistogenes songorica, a xerophytic desert grass[J]. Plant Omics, 2015, 8(6):485-492.
[7] SUN Y S, HUANG H J, JIANG J, et al. Improved salt tolerance of Populus davidiana×P. bolleana overexpressed LEA from Tamarix androssowii[J]. Journal of Forestry Research, 2014, 25(4):813-818. doi: 10.1007/s11676-014-0529-z
[8] 罗子敬, 孙宇涵, 卢楠, 等.杨树耐盐机制及转基因研究进展[J].核农学报, 2017, 31(3):482-492. http://d.old.wanfangdata.com.cn/Periodical/hnxb201703009 LUO Z J, SUN Y H, LU N, et al. Research advances on salt-tolerance mechanism and genetic transformation of poplar[J]. Journal of Nuclear Agricultural Sciences, 2017, 31(3):482-492. http://d.old.wanfangdata.com.cn/Periodical/hnxb201703009
[9] JAMBUNATHAN N. Determination and detection of reactive oxygen species (ROS), lipid peroxidation, and electrolyte leakage in plants[J]. Methods in Molecular Biology, 2010, 639(1):291-297. http://cn.bing.com/academic/profile?id=f663812894f822f83ae081dfbeaf312b&encoded=0&v=paper_preview&mkt=zh-cn
[10] 倪妍妍, 常二梅, 刘建锋, 等.不同树龄侧柏接穗光合生理的比较研究[J].西北林学院学报, 2017, 32(1):19-24. doi: 10.3969/j.issn.1001-7461.2017.01.03 NI Y Y, CHANG E M, LIU J F, et al. Comparison on photosynthetic physiology in various age scions of Platycladus orientalis[J]. Chinese Academy of Forestry, 2017, 32(1):19-24. doi: 10.3969/j.issn.1001-7461.2017.01.03
[11] 尤鑫, 龚吉蕊.叶绿素荧光动力学参数的意义及实例辨析[J].西部林业科学, 2012, 41(5):90-94. doi: 10.3969/j.issn.1672-8246.2012.05.017 YOU X, GONG J R. Significance and application of chlorophyll fluorescence dynamics process parameters[J]. Journal of West China Forestry Science, 2012, 41(5):90-94. doi: 10.3969/j.issn.1672-8246.2012.05.017
[12] 王智明, 张峰举, 许兴.植物耐盐生理生化指标研究进展[J].湖北农业科学, 2014, 53(7):1493-1496. doi: 10.3969/j.issn.0439-8114.2014.07.003 WANG Z M, ZHANG F J, XU X. Advances on physiological and biochemical indexes of salt tolerance in plant[J]. Hubei Agricultural Sciences, 2014, 53(7):1493-1496. doi: 10.3969/j.issn.0439-8114.2014.07.003
[13] WANG L, ZHANG J L, WANG D, et al. Assessment of salt tolerance in transgenic potato carrying AtNHX1 gene[J].Crop Science, 2013, 53(6):2643-2651. doi: 10.2135/cropsci2013.03.0179
[14] WANG F W, WANG C, SUN Y, et al. Overexpression of vacuolar proton pump ATPase (V-H+ -ATPase) subunits B, C and H confers tolerance to salt and saline-alkali stresses in transgenic alfalfa (Medicago sativa L.)[J]. Journal of Integrative Agriculture, 2016, 15(10):2279-2289. doi: 10.1016/S2095-3119(16)61399-0
[15] 才华, 宋婷婷, 张大洋. rd29A和CaMV-35S启动子调控转AtDREB2A苜蓿耐碱性分析[J].东北农业大学学报, 2016, 47(9):16-23. doi: 10.3969/j.issn.1005-9369.2016.09.003 CAI H, SONG T T, ZHANG D Y. Alkaline tolerance analysis of transgenic alfalfa with AtDREB2A gene regulated by rd29A and CaMV-35S promoter[J]. Journal of Northeast Agricultural University, 2016, 47(9):16-23. doi: 10.3969/j.issn.1005-9369.2016.09.003
[16] DALAL M, TAYAL D, CHINNUSAMY V, et al. Abiotic stress and ABA-inducible group 4 LEA from Brassica napus plays a key role in salt and drought tolerance[J]. Journal of Biotechnology, 2008, 139(2):137-145. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=74b85cd7e966d3682afc4169b80ab6cf
[17] YU J, LAI Y, WU X, et al. Overexpression of OsEm1 encoding a group Ⅰ LEA protein confers enhanced drought tolerance in rice[J]. Biochemical and Biophysical Research Communications, 2016, 478(2):703-709. doi: 10.1016/j.bbrc.2016.08.010
[18] 周珩, 郭世荣, 邵慧娟, 等.等渗NaCl和Ca(NO3)2胁迫对黄瓜幼苗生长和生理特性的影响[J].生态学报, 2014, 34(7):1880-1890. http://d.old.wanfangdata.com.cn/Periodical/stxb201407028 ZHOU Y, GUO S R, SHAO H J, et al. Effects of iso-osmotic Ca(NO3)2 and NaCl stress on growth and physiological characteristics of cucumber seedlings[J]. Acta Ecologica Sinica, 2014, 34(7):1880-1890. http://d.old.wanfangdata.com.cn/Periodical/stxb201407028
[19] 卢闯, 逄焕成, 赵长海, 等.水分胁迫下施磷对潮土玉米苗期叶片光合速率、保护酶及植株养分含量的影响[J].中国生态农业学报, 2017, 25(2):239-246. http://d.old.wanfangdata.com.cn/Periodical/stnyyj201702011 LU C, FENG H C, ZHAO C H, et al. Effect of phosphorus on leaf net photosynthesis, protective enzyme activity and nutrient uptake of maize at seedling stage in fluvo-aquic soils under water stress[J]. Chinese Journal of Eco-Agriculture, 2017, 25(2):239-246. http://d.old.wanfangdata.com.cn/Periodical/stnyyj201702011
[20] 许大全.光合作用气孔限制分析中的一些问题[J].植物生理学通讯, 1997, 33(4):241-244. doi: 10.1063-1.1913612/ XU D Q. Some problems in stomatal limitation analysis of photosynthesis[J]. Plant Physiology Communications, 1997, 33(4): 241-244. doi: 10.1063-1.1913612/
[21] MAXWELL K, JOHNSON G. Chlorophyll fluorescence: a practical guide[J]. Journal of Experimental Botany, 2000, 51(345):659-668. doi: 10.1093/jexbot/51.345.659
[22] 寇江涛, 康文娟, 苗阳阳, 等.外源EBR对NaCl胁迫下紫花苜蓿幼苗微量元素吸收及叶绿素荧光动力学参数的影响[J].中国生态农业学报, 2016, 24(3):345-355. http://d.old.wanfangdata.com.cn/Periodical/stnyyj201603009 KOU J T, KANG W J, MIAO Y Y, et al. Effect of exogenous 2, 4-epibrassinolide on trace element absorption and chlorophyll fluorescence of Medicago sativa L. seedlings under NaCl stress[J]. Chinese Journal of Eco-Agriculture, 2016, 24(3):345-355. http://d.old.wanfangdata.com.cn/Periodical/stnyyj201603009
[23] 徐焕文, 刘宇, 姜静, 等.盐胁迫对白桦光合特性及叶绿素荧光参数的影响[J].西南林业大学学报, 2015, 35(4):21-26. http://d.old.wanfangdata.com.cn/Periodical/xnlxyxb201504004 XU H W, LIU Y, JIANG J, et al. Changes of photosynthetic characteristics and chlorophyll fluorescence parameters of Betula platyphylla under salt stress[J]. Journal of Southwest Forestry University, 2015, 35(4):21-26. http://d.old.wanfangdata.com.cn/Periodical/xnlxyxb201504004
[24] 李旭新, 刘炳响, 郭智涛, 等. NaCl胁迫下黄连木叶片光合特性及快速叶绿素荧光诱导动力学曲线的变化[J].应用生态学报, 2013, 24(9):2479-2484. http://d.old.wanfangdata.com.cn/Periodical/yystxb201309012 LI X X, LIU B X, GUO Z T, et al. Effects of NaCl stress on photosynthesis characteristics and fast chlorophyll fluorescence induction dynamics of Pistacia chinensis leaves[J]. Chinese Journal of Applied Ecology, 2013, 24(9):2479-2484. http://d.old.wanfangdata.com.cn/Periodical/yystxb201309012
[25] 张守仁, 高荣孚.光胁迫下杂种杨无性系光合生理生态特性的研究[J].植物生态学报, 2000, 24(5):528-533. doi: 10.3321/j.issn:1005-264X.2000.05.004 ZHANG S R, GAO R F. Ecophysiological characteristics of photosynthesis of hybrid poplar clones under light stress[J]. Acta Phytoecologica Sinica, 2000, 24(5):528-533. doi: 10.3321/j.issn:1005-264X.2000.05.004
[26] 施征, 史胜青, 姚洪军, 等.植物线粒体中活性氧的产生及其抗氧化系统[J].北京林业大学学报, 2009, 31(1):150-154. doi: 10.3321/j.issn:1000-1522.2009.01.026 SHI Z, SHI S Q, YAO H J, et al. Production of ROS and its antioxidant system in plant mitochondria[J]. Journal of Beijing Forestry University, 2009, 31(1):150-154. doi: 10.3321/j.issn:1000-1522.2009.01.026
[27] 戴海芳, 武辉, 阿曼古丽·买买提阿力, 等.不同基因型棉花苗期耐盐性分析及其鉴定指标筛选[J].中国农业科学, 2014, 47(7):1290-1300. doi: 10.3864/j.issn.0578-1752.2014.07.005 DAI H F, WU H, MAIMAITIALI A, et al. Analysis of salt-tolerance and determination of salt-tolerant evaluation indicators in cotton seedlings of different genotypes[J]. Scientia Agricultura Sinica, 2014, 47(7):1290-1300. doi: 10.3864/j.issn.0578-1752.2014.07.005
[28] 李扬, 刘关君, 曲春浦, 等.西伯利亚蓼PsLEA基因的克隆及在NaHCO3胁迫下的表达[J].分子植物育种, 2010, 8(2):276-282. http://d.old.wanfangdata.com.cn/Periodical/fzzwyz201002011 LI Y, LIU G J, QU C P, et al. Cloning of PsLEA gene from Polygonum sibiricum Laxm. and its expression under NaHCO3 stress[J]. Molecular Plant Breeding, 2010, 8(2):276-282. http://d.old.wanfangdata.com.cn/Periodical/fzzwyz201002011
-
期刊类型引用(2)
1. 王阳,王伟,姜静,顾宸瑞,杨蕴力. 转基因小黑杨根际土壤微生物群落特征研究. 南京林业大学学报(自然科学版). 2023(01): 199-208 .  百度学术
百度学术
2. 程贝贝,陈胜艳,岳莉然. NaHCO_3胁迫对紫根水葫芦形态学指标和光合参数的影响. 广西植物. 2020(12): 1781-1789 .  百度学术
百度学术
其他类型引用(4)




 下载:
下载: