Foliar condensate absorption capacity of four typical plant species and their physiological responses to water in the Mu Us Sandy Land of northwestern China
-
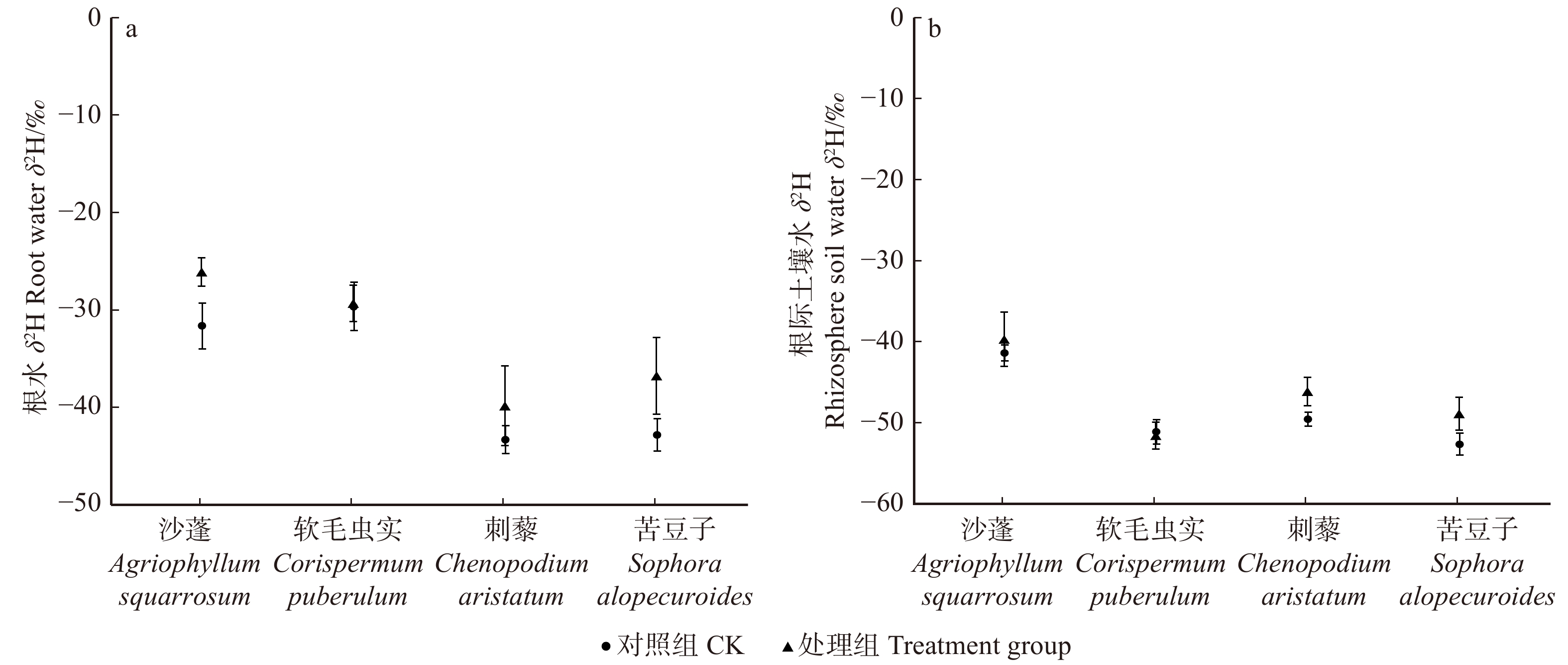
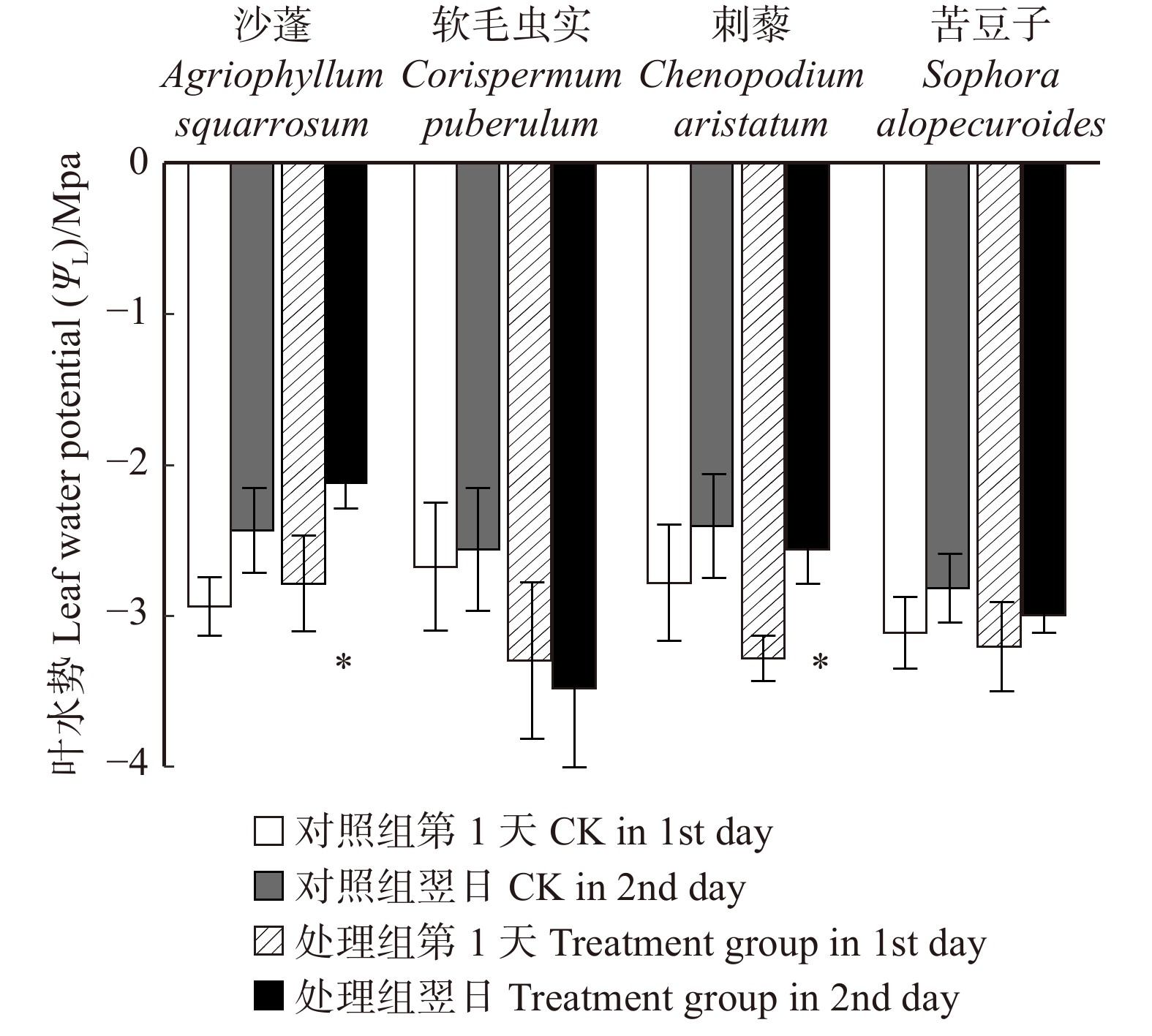
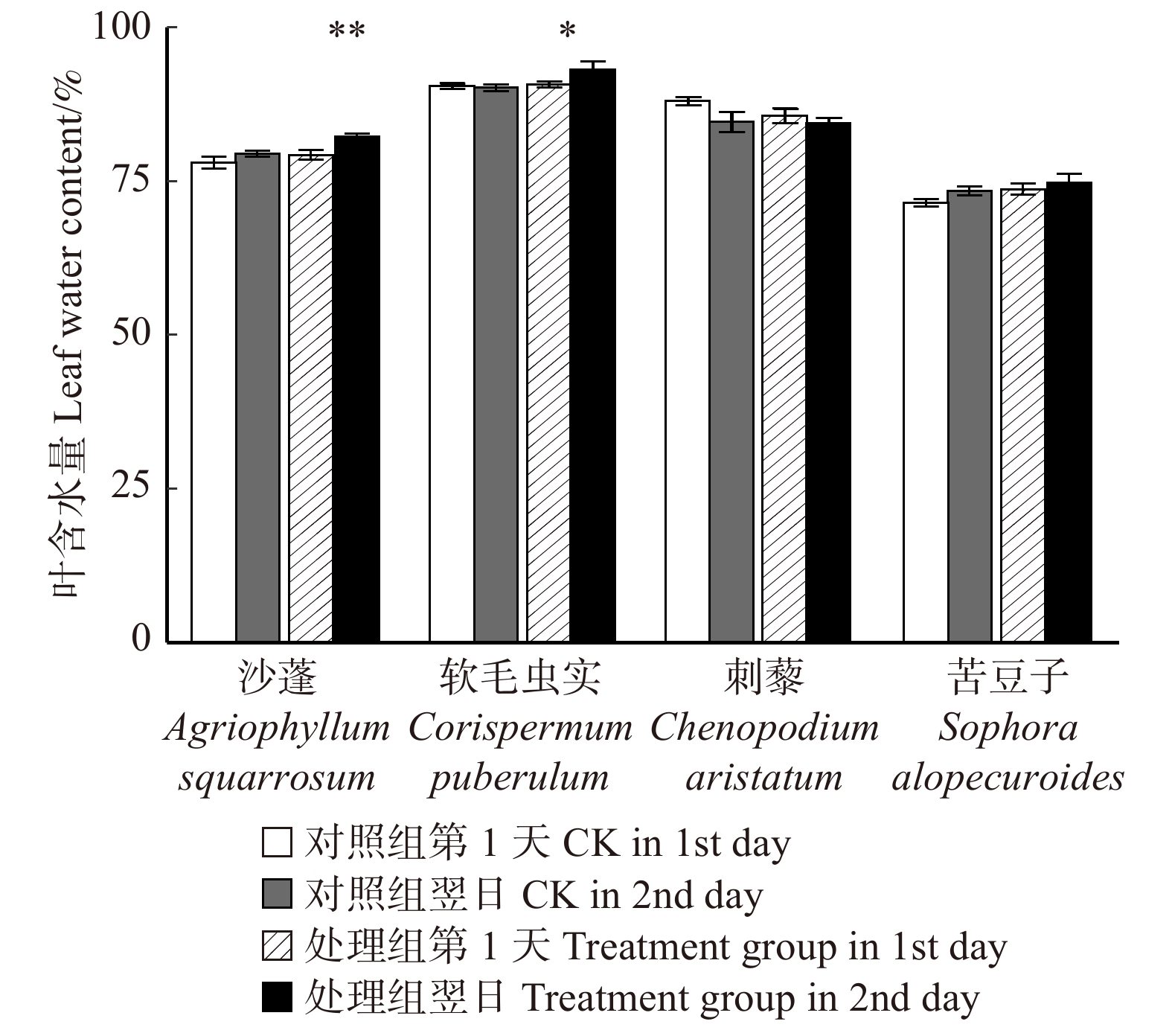
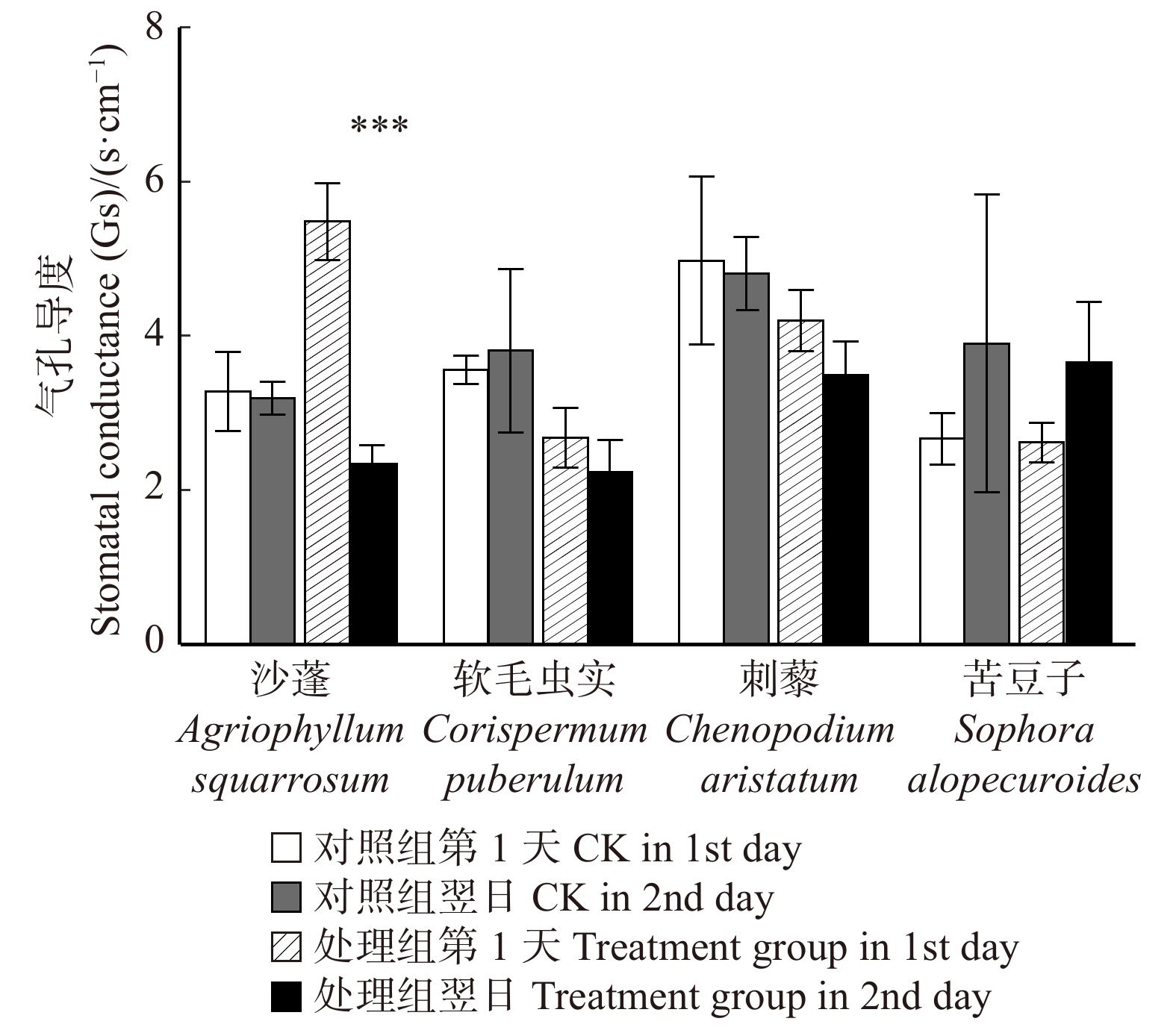
摘要:目的 明确毛乌素沙地4种典型植物沙蓬、软毛虫实、刺藜和苦豆子的叶片凝结水吸收能力,阐明植物叶片对凝结水浸润的水分生理响应。方法 将受试植物置于用高丰度氘水配置的人工标记凝结水环境中,进行凝结水浸润处理,通过比较处理组和对照组植物叶水、根水及根际土壤水的稳定氢同位素丰度变化,确定受试植物叶片是否具有吸水能力,示踪叶片吸收凝结水后,是否将水分转移到植物根系及根际土壤之中;使用露点水势仪、电子天平及气孔计,测定受试植物处理前后的叶水势、叶片含水量和气孔导度变化,了解受试植物对凝结水浸润的水分生理响应。结果 (1)高丰度氘标记凝结水浸润后,处理组4种受试植物的叶水δ2H(20‰ ~ 100‰)均显著高于对照组(−25‰ ~ −15‰),而根水(−45‰ ~ −30‰)及根际土壤水(−50‰ ~ −40‰)则与对照组无显著差异;(2)经过凝结水浸润试验处理,沙蓬的叶水势升高23.81%,叶含水量升高2.94%,气孔导度降低57.40%;软毛虫实的叶含水量升高了2.45%,叶水势和气孔导度无显著变化;刺藜的叶水势升高了21.95%,气孔导度和叶含水量无显著变化;苦豆子的叶水势、叶含水量和气孔导度均无显著变化。结论 毛乌素沙地4种典型植物叶片均具有凝结水吸收能力,叶片吸收的水分未被发现转移至根部或根际土壤。沙蓬、软毛虫实、刺藜通过叶片吸水显著改善了自身水分生理状态,这可能是其适应沙地严酷水分条件的重要水分利用机制,有助于植物存活,而苦豆子叶片对凝结水浸润无明显响应,不能有效利用叶片吸水改变其水分生理状态。Abstract:Objective In this study, we examined Agriophyllum squarrosum, Corispermum puberulum, Chenopodium aristatum, and Sophora alopecuroides in the Mu Us Desert of northwestern China to explore the ability of leaf condensate absorption and their physiological responses to water.Method We determined whether the condensate can be absorbed by the leaves of four plant species, and whether the absorbed water can be transported to the root and rhizosphere soil by dew covered experiments and stable isotope tracer technology to contrast the δ2H values of samples in the leaves, roots and rhizosphere soil from the treatment and control. Meanwhile, we measured leaf water potential (ΨL), leaf water content (wL), and stomatal conductance (Gs) before and after dew treatment by dew point water potential meter, electronic balance and plant porometer in order to evaluate the effects of foliar condensate absorption on the species.Result (1) After the deuterium labelled condensate treatment, the δ2H values in samples of leaves (20‰−100‰) in the treatment group of four plant species were significantly higher than control (−25‰− −15‰); the δ2H values in samples of root(−45‰ − −30‰) and rhizosphere soil (−50‰ − −40‰) in the treatment group did not change significantly compared with control. (2) After the dew treatment, ΨL, wL of A. squarrosum increased by 23.81%, 2.49%, and Gs of it decreased by 57.40%, respectively; wL of C. puberulum increased by 2.45%, but the ΨL and Gs were not change significantly; ΨL of C. aristatum increased by 21.95%, but the wLand Gs were not change obviously; for S. alopecuroides, there were no significant differences in ΨL, wL and Gs.Conclusion We find that all of the four plant species can absorb condensate through leaves, but the water cannot be transported to the root and rhizosphere soil. Agriophyllum squarrosum, Corispermum puberulum and Chenopodium aristatum could improve their water physiological state through water absorption. It may be an important water use strategy for them to adapt to drought environment, for this helps the plants to survive. However, Sophora alopecuroides does not significantly respond to foliar condensate absorption and also could not improve its water physiological state through this process.
-
Keywords:
- condensate /
- foliar water absorption /
- physiological response /
- water use strategy /
- desert plant
-
各种人类活动,如采矿、化石燃料燃烧、工业废气排放、以及过量使用化肥,源源不断地将重金属带入环境,给自然环境造成严重的生态毒理效应[1-2]。由重金属离子造成的包括大气、水体和土壤在内的环境污染统称为重金属污染,与有机质污染相比,重金属污染具有长期性、不可逆性和隐蔽性等特征[3]。通常,按重金属是否参与植物体内的各种新陈代谢反应,将其分为必需元素和非必需元素。其中,锌(Zn)作为一种植物必需的微量元素,能通过直接或间接参与植物体内的光合作用、呼吸作用以及碳水化合物的合成、运转等过程的方式,来促进植物的生长发育和提高其抗逆能力[4-5]。然而,当环境中的Zn含量超过某一临界阈值时,就会对植物产生毒害作用,如分解叶片内的叶绿素、降低光合作用速率和造成缺铁性失绿等,轻则使植物代谢过程发生紊乱、生长状况不良,重则导致植物死亡[6-7]。
与受到昆虫取食、光照强度、干旱等生物或非生物因子刺激时的效果一致,重金属胁迫能通过激发植物体内的茉莉酸信号途径和防御基因表达来间接地引发植物的诱导防御反应,从而使植物产生和积聚一系列有毒、抗营养和抗消化的新陈代谢产物,并对植食昆虫正常的生长、发育和繁殖造成抑制或干扰作用[8-10]。例如,Cabot等[11]研究发现镉胁迫能诱导拟南芥(Arabidopsis thaliana)激活茉莉酸-乙烯信号途径中防卫素基因(PDF1.2)的表达。Wang等[12]研究发现铅胁迫能显著提高沉水植物苦草(Vallisneria natans)体内的总酚和黄酮含量。然而,依据近来提出的“权衡”假说,为了减弱重金属造成的毒害作用,植物势必会降低需要消耗能量的化学防御水平,从而选择将更多的能量用于提高植物对重金属的解毒能力,如大量合成抗氧化酶、增强对重金属的转运和隔离能力[13-14]。目前,植物化学防御在重金属胁迫下的响应趋势尚不明确,但其可能受植物和重金属种类,以及胁迫浓度和时间的影响。
作者前期研究发现,Zn胁迫下的银中杨(Populus alba ‘Berolinensis’)能抑制叶部害虫舞毒蛾(Lymantria dispar)幼虫的生长发育[15],但由于叶片中富集的Zn也能对舞毒蛾产生有效的元素防御,因而尚不清楚Zn胁迫是否能对银中杨的化学防御产生影响。本研究用不同含量的Zn处理盆栽1年生银中杨的土壤,通过测定其生长发育状况、叶片内营养物质和次生物质含量以及蛋白酶抑制剂活性,以期弄清Zn胁迫对银中杨生长发育的影响,以及银中杨化学防御在Zn胁迫下的响应趋势,为重金属污染地区的植物虫害防治提供基础理论依据。
1. 材料与方法
1.1 供试植物及重金属处理
2015年4月末,于黑龙江平山森林植物试种苗圃,将1年生银中杨插扦苗种植于塑料花盆中,花盆规格为25 cm×28 cm(高×直径)。定期浇水除草,待树苗生长2个月后,将长势基本一致的苗木分成4组,每组50株。然后,用ZnSO4水溶液处理其中3组苗木生长的土壤,使土壤中的Zn2+含量分别为300、500和700 mg/kg,其标记代码依次为Zn300、Zn500和Zn700。最后1组加等量的水作为对照,标记代码为CK。在Zn胁迫后30、40、50 d,于每组中随机选择9株苗木,设为3个重复,然后将每个重复中的所有叶片采摘下来用冰盒带回实验室,并放入-40 ℃低温冰箱中保存,用于测定营养物质和次生物质含量以及蛋白酶抑制剂活性。Zn在土壤中的质量分数设计依据土壤环境质量标准,农林生产和植物正常生长的土壤临界值进行确定[16]。
1.2 生长发育测定
在Zn胁迫2个月后,于每组中随机选择9株银中杨进行整株采样。清理干净后,整株苗木按根、茎、叶分为3部分,然后测定每株苗木的株高、地径(植物土迹处的直径)和根长,以及测定根、茎和叶的鲜质量。接着将每株苗木的根、茎、叶在70 ℃下烘干至恒质量,测定其根、茎和叶的干质量。
1.3 抗虫物质含量测定
参照Bradford[17]和Nelson[18]的方法分别对叶片中蛋白质和可溶性糖进行提取和测定。参照Zhishen等[19]和任琴等[20]的方法分别对叶片中黄酮和木质素进行提取和测定。参照张健等[21]的方法对叶片中胰蛋白酶抑制剂(TI)和胰凝乳蛋白酶抑制剂(CI)进行提取和测定。
1.4 数据处理与分析
采用SPSS19.0软件统计数据的平均值和标准差(n=3),并采用该软件的one-way ANOVA,以LSD(最小显著法)在0.05水平下分析各组之间银中杨生长发育状况的差异显著性,以及分析相同胁迫时间下的各组之间叶片中营养物质含量、次生物质含量或蛋白酶抑制剂活性的差异显著性。
2. 结果与分析
2.1 Zn胁迫对银中杨生长发育的影响
由图 1和表 1可知,Zn胁迫能使银中杨的生长参数(株高、根长和地径)和生物量参数(根、茎、叶的鲜质量和干质量)均显著低于对照(P<0.05),且其具有一定的浓度效应。说明Zn胁迫能抑制银中杨的生长发育,对其产生毒害作用。
![]() 图 1 Zn胁迫对银中杨株高、根长和地径的影响图中数据均为平均值±标准差(n=3)。不同小写字母表示不同处理之间差异显著(P<0.05)。Figure 1. Effects of Zn stress on plant height (A), root length (B) and ground diameter (C) of Populus alba 'berolinensis' seedlingsData in figure are means±SD (n=3). Different lowercases mean there is significant differences among the treatments at P<0.05 level.表 1 Zn胁迫对银中杨根、茎、叶鲜质量和干质量的影响Table 1. Effects of Zn stress on the fresh and dry mass of root, stem and leaf of Populus alba 'berolinensis' seedlings
图 1 Zn胁迫对银中杨株高、根长和地径的影响图中数据均为平均值±标准差(n=3)。不同小写字母表示不同处理之间差异显著(P<0.05)。Figure 1. Effects of Zn stress on plant height (A), root length (B) and ground diameter (C) of Populus alba 'berolinensis' seedlingsData in figure are means±SD (n=3). Different lowercases mean there is significant differences among the treatments at P<0.05 level.表 1 Zn胁迫对银中杨根、茎、叶鲜质量和干质量的影响Table 1. Effects of Zn stress on the fresh and dry mass of root, stem and leaf of Populus alba 'berolinensis' seedlings处理组Treatment 根质量Root mass/(g·plant-1) 茎质量Stem mass/(g· plant-1) 叶质量Leaf mass/(g· plant-1) 鲜质量Fresh mass 干质量Dry mass 鲜质量Fresh mass 干质量Dry mass 鲜质量Fresh mass 干质量Dry mass CK 4.98±0.59a 3.77±0.29a 14.93±0.81a 7.58±0.51a 19.54±2.01a 9.09±0.96a Zn300 3.92±0.19b 3.06±0.34b 13.66±0.19b 6.73±0.1b 16.77±0.15b 7.02±0.18b Zn 500 3.53±0.37b 2.81±0.34b 13.60±0.27b 6.47±0.07b 15.75±0.67b 6.39±0.36b Zn 700 3.46±0.33b 2.64±0.28b 11.24±1.33b 5.26±0.67c 15.53±0.53b 6.36±0.3b 注:表中数据均为平均值±标准差(n=3)。同列不同小写字母表示不同处理之间差异显著(P<0.05)。CK、Zn300、Zn500、Zn700分别代表土壤中的Zn处理含量为0、300、500和700 mg/kg。下同。Notes: data in table are means±SD (n=3). Different lowercases within the same column mean there is significant differences among the treatments at P<0.05 level. CK, Zn300, Zn500, Zn700 represent the Zn contents of 0, 300, 500, 700 mg/kg, respectively (the same below). 2.2 Zn胁迫对叶片内营养物质含量的影响
Zn胁迫下叶片中的蛋白质和可溶性糖含量分别见图 2。由图 2可知,在Zn胁迫后30、40和50 d,各含量处理均使银中杨叶片中的蛋白质和可溶性糖含量显著低于对照(P<0.05),但处理组之间的蛋白质含量均差异不显著(P>0.05),而可溶性糖含量均表现为随胁迫含量的增高而增高。
![]() 图 2 Zn胁迫对银中杨叶片内蛋白质(A)和可溶性糖(B)含量的影响图中数据均为平均值±标准差(n=3)。不同小写字母表示相同胁迫时间下各组之间差异显著(P<0.05)。下同。Data in figure are means±SD (n=3).Figure 2. Effects of Zn stress on the protein (A) and soluble sugar (B) content in leaves of Populus alba 'berolinensis' seedlingsDifferent lowercases within the same stress duration mean there is significant differences among the treatments at P<0.05 level. The same below.
图 2 Zn胁迫对银中杨叶片内蛋白质(A)和可溶性糖(B)含量的影响图中数据均为平均值±标准差(n=3)。不同小写字母表示相同胁迫时间下各组之间差异显著(P<0.05)。下同。Data in figure are means±SD (n=3).Figure 2. Effects of Zn stress on the protein (A) and soluble sugar (B) content in leaves of Populus alba 'berolinensis' seedlingsDifferent lowercases within the same stress duration mean there is significant differences among the treatments at P<0.05 level. The same below.2.3 Zn胁迫对叶片内次生物质含量的影响
Zn胁迫下叶片中的黄酮和木质素含量见图 3。由图 3可知,在Zn胁迫后30、40和50 d,各含量处理均诱使银中杨叶片内的黄酮和木质素含量显著高于对照(P<0.05),但诱导效果随Zn胁迫含量的增高而降低。说明Zn胁迫能诱导银中杨增强叶片内的酚类代谢,且低含量胁迫诱导效果最强。
2.4 Zn胁迫对叶片内蛋白酶抑制剂活性的影响
Zn胁迫下叶片中的TI和CI活性见图 4。由图 4可见,在Zn胁迫后30、40和50 d,各含量处理均使银中杨叶片内的TI和CI活性显著低于对照,且高含量胁迫下的TI和CI活性均显著低于中含量和低含量胁迫(P<0.05)。
3. 结论与讨论
重金属胁迫下,敏感型植物容易遭受其带来的毒害作用,造成植物体内各种生理生化过程发生紊乱,例如光合作用减弱,膜通透性改变和细胞器结构破坏,从而严重影响植物的正常生长发育[22-23]。即使耐性较强的植物,为了在重金属胁迫下维持细胞内稳态的平衡,必将会消耗植物生长过程中的有效能量,最终使植物生长发育受阻[24]。本研究发现Zn胁迫能使银中杨的生长参数(株高、根长和地径)和生物量参数(根、茎、叶的鲜质量和干质量)均显著低于对照(P<0.05),且其具有一定的浓度效应。说明Zn作为植物生长的必需元素,在其含量超过一定阈值时,也能抑制银中杨的生长发育,对银中杨产生毒害作用。此外,重金属胁迫下,生长发育受抑制也在万寿菊(Tagetes erecta)[25]、钻天杨(Populus nigra)[26]和美洲黑杨(P. deltoides)[27]等草本或木本植物中被报道,表明植物生长发育状况是表征重金属胁迫对植物毒害作用的一项重要生理指标。
取食是植食性昆虫获取自身生长发育所需能源的唯一途径,当植物中的营养物质含量低于昆虫最适量范围时,就会造成昆虫营养不良和必需元素缺乏,从而影响昆虫的取食和正常生长发育[28-29]。因此,植物体内的营养物质含量能充当一种潜在的抗虫因素。本研究发现在Zn胁迫后30、40和50 d,各含量处理均使银中杨叶片中的蛋白质和可溶性糖含量显著低于对照(P<0.05)。说明生长基质中过量的Zn能导致银中杨叶片内营养物质缺乏。可以推断,蛋白质和可溶性糖含量降低是Zn胁迫银中杨能抑制害虫舞毒蛾生长发育的重要原因之一。此外,本研究还发现在Zn胁迫后的各时间段,处理组之间的蛋白质含量均差异不显著(P>0.05),可溶性糖含量均表现为随胁迫含量的增高而增高。说明蛋白质和可溶性糖作为一类渗透调节物质[30],能协助银中杨部分缓解高含量Zn胁迫对其造成的毒害作用。
次生代谢产物作为植物体内一类结构复杂、种类繁多的有机物质,虽不直接参与植物的生长发育,但能抑制植食昆虫对食物的消化利用、干扰其交配行为以及吸引天敌,在植物抵御昆虫侵袭过程中发挥着重要作用,是衡量植物抗虫性能的一个重要生理指标[31-32]。本研究发现Zn各含量胁迫均诱使银中杨叶片内的黄酮和木质素含量在各时间段显著高于对照(P<0.05),但诱导效果随Zn胁迫含量的增高而降低。说明Zn胁迫能诱导银中杨增强叶片内的酚类代谢,且低含量胁迫诱导效果最强。银中杨在Zn胁迫下,叶片内黄酮和木质素的大量合成,可能是因为黄酮和木质素能参与银中杨对Zn的解毒,如提高银中杨的抗氧化能力和对Zn的螯合能力。
TI和CI是两种与植物抗虫性密切相关的一类小分子蛋白质,能与昆虫消化道内的蛋白消化酶形成酶抑制剂复合物,从而降低昆虫对食物中蛋白质的消化能力[33-34]。张凯等[35]在研究重金属胁迫对杨树(P. simonii×P. nigra)叶片中防御蛋白活性的影响时发现,Cu或Cd胁迫均会促使TI和CI活性迅速升高。说明植物叶片内蛋白酶抑制剂能积极响应外部环境中的重金属胁迫。然而,本研究发现,Zn各含量胁迫均使银中杨叶片内的TI和CI活性在各时间段显著低于对照(P<0.05)。说明由Zn胁迫在银中杨叶片内引起的氧化压力可能对TI和CI造成了氧化损伤,TI和CI对重金属的响应趋势取决于所研究的重金属和植物种类。
重金属胁迫下,植物可能同时存在着两种抗虫防御机制:重金属介导的植物化学防御和重金属元素的直接防御[36]。二者均有助于提高植物对植食性昆虫的抗性,且根据最近提出的“联合”假说[37],重金属可以与化学防御物质产生相加或协同效应,造成植物的防御反应全面提升。因此,依据本研究的结果和先前研究的银中杨叶片对Zn的富集结果[38],可表明作者前期研究发现的毒理结果[15],即取食Zn胁迫下银中杨的叶片后,舞毒蛾幼虫生长发育减缓,可归结为重金属介导的植物化学防御和叶片中富集重金属的元素防御的共同作用。
综上所述,生长基质中过量的Zn会抑制银中杨的生长发育,且能影响银中杨的化学防御水平。然而,银中杨叶片内与抗虫相关物质的含量或活性对Zn胁迫的响应趋势并不一致,具体表现为营养物质含量减少、次生物质含量增多、蛋白酶抑制剂活性减弱。
-
-
[1] 蒋瑾, 王康富, 张维静. 沙地凝结水及在水分平衡中作用的研究[J]. 干旱区研究, 1993, 10(2):1−9. Jiang J, Wang K F, Zhang W J. Study on condensation water of sandy land and its role in water balance[J]. Arid Zone Research, 1993, 10(2): 1−9.
[2] Zangvil A. Six years of dew observations in the Negev Desert, Israel[J]. Journal of Arid Environments, 1996, 32(4): 361−371. doi: 10.1006/jare.1996.0030.
[3] Kalthoffa N, Fiebig-Wittmaack M, Meiβner C, et al. The energy balance, evapo-transpiration and nocturnal dew deposition of an arid valley in the Andes[J]. Journal of Arid Environments, 2006, 65(3): 420−443. doi: 10.1016/j.jaridenv.2005.08.013.
[4] Malek E, McCurdy G, Giles B. Dew contribution to the annual water balances in semi-arid desert valleys[J]. Journal of Arid Environments, 1999, 42(2): 71−80. doi: 10.1006/jare.1999.0506.
[5] 郭晓楠, 查天山, 贾昕, 等. 典型沙生灌木生态系统凝结水量估算[J]. 北京林业大学学报, 2016, 38(10):80−87. Guo X N, Zha T S, Jia X, et al. Estimation of dewfall amount in a typical desert shrub ecosystem[J]. Journal of Beijing Forestry University, 2016, 38(10): 80−87.
[6] Scanlon B R, Milly P C D. Water and heat fluxes in desert soils(2): numerical simulations[J]. Water Resources Research, 1994, 30(3): 721−734. doi: 10.1029/93WR03252
[7] 曾亦键, 万力, 王旭升, 等. 浅层包气带地温与含水量昼夜动态的实验研究[J]. 地学前缘, 2006, 13(1):52−57. doi: 10.3321/j.issn:1005-2321.2006.01.008. Zeng Y J, Wan L, Wang X S, et al. An experimental study of day and night trends of soil temperature and moisture in the shallow unsaturated zone[J]. Earth Science Frontiers, 2006, 13(1): 52−57. doi: 10.3321/j.issn:1005-2321.2006.01.008.
[8] Jacobs A F G, Heusinkveld B G, Berkowicz S M. Dew deposition in a desert system: a simple simulation model[J]. Journal of Arid Environments, 1999, 42(3): 211−222. doi: 10.1006/jare.1999.0523
[9] Baguskas S A, King J Y, Fischer D T, et al. Impact of fog drip versus fog immersion on the physiology of bishop pine saplings[J]. Functional Plant Biology, 2017, 44(3): 339−350. doi: 10.1071/FP16234.
[10] 龚雪伟. 荒漠木本植物光合器官吸收冠层凝结水机理探究[D]. 乌鲁木齐: 新疆大学, 2017. Gong X W. A probe into the mechanisms of canopy dew uptake by photosynthetic organs of desert trees: based on molecular, cellular and physiological perspectives[D]. Urumqi: Xinjiang University, 2017.
[11] Wang X H, Xiao H L, Cheng Y B, et al. Leaf epidermal water-absorbing scales and their absorption of unsaturated atmospheric water in Reaumuria soongorica, a desert plant from the northwest arid region of China[J]. Journal of Arid Environments, 2016, 128: 17−29. doi: 10.1016/j.jaridenv.2016.01.005.
[12] Wang X H, Xiao H L, Ren J, et al. An ultrasonic humidification fluorescent tracing method for detecting unsaturated atmospheric water absorption by the aerial parts of desert plants[J]. Journal of Arid Land, 2016, 8(2): 272−283. doi: 10.1007/s40333-015-0018-z.
[13] Yan X, Zhou M X, Dong X C, et al. Molecular mechanisms of foliar water uptake in a desert tree[J]. AoB Plants, 2015, 7: 129.
[14] 庄艳丽, 赵文智. 荒漠植物雾冰藜和沙米叶片对凝结水响应的模拟实验[J]. 中国沙漠, 2010, 30(5):1068−1074. Zhuang Y L, Zhao W Z. Experimental study of effects of artificial dew on Bassia dasyphylla and Agriophyllum squarrosum[J]. Journal of Desert Research, 2010, 30(5): 1068−1074.
[15] Stone E C. Dew as an ecological factor(II): the effect of artificial dew on the survival of Pinus ponderosa and associated species[J]. Ecology, 1957, 38(3): 414−422. doi: 10.2307/1929884.
[16] Martin C E, Willert D J. Leaf epidermal hydathodes and the ecophysiological consequences of foliar water uptake in species of Crassula from the Namib Desert in Southern Africa[J]. Plant Biology, 2000, 2(2): 229−242. doi: 10.1055/s-2000-9163.
[17] Hill A J, Dawson T E, Shelef O, et al. The role of dew in Negev desert plants[J]. Oecologia, 2015, 178(2): 317−327. doi: 10.1007/s00442-015-3287-5.
[18] Goldsmith G R, Matzke N J, Dawson T E. The incidence and implications of clouds for cloud forest plant water relations[J]. Ecology Letters, 2013, 16(3): 307−314. doi: 10.1111/ele.12039.
[19] Fu P L, Liu W J, Fan Z X, et al. Is fog an important water source for woody plants in an Asian tropical karst forest during the dry season?[J]. Ecohydrology, 2016, 9(6): 964−972. doi: 10.1002/eco.1694.
[20] 郑玉龙, 冯玉龙. 西双版纳地区附生与非附生植物叶片对雾水的吸收[J]. 应用生态学报, 2006, 17(6):977−981. doi: 10.3321/j.issn:1001-9332.2006.06.005. Zheng Y L, Feng Y L. Fog water absorption by the leaves of epiphytes and non-epiphytes in Xishuangbanna[J]. Chinese Journal of Applied Ecology, 2006, 17(6): 977−981. doi: 10.3321/j.issn:1001-9332.2006.06.005.
[21] 郑新军, 李嵩, 李彦. 准噶尔盆地荒漠植物的叶片水分吸收策略[J]. 植物生态学报, 2011, 35(9):893−905. doi: 10.3724/SP.J.1258.2011.00893 Zheng X J, Li S, Li Y. Leaf water uptake strategy of desert plants in the Junggar Basin, China[J]. Chinese Journal of Plant Ecology, 2011, 35(9): 893−905. doi: 10.3724/SP.J.1258.2011.00893
[22] Vitarelli N C, Riina R, Cassino M F, et al. Trichome-like emergences in Croton of Brazilian highland rock outcrops: evidences for atmospheric water uptake[J]. Perspectives in Plant Ecology, Evolution and Systematics, 2016, 22: 23−35. doi: 10.1016/j.ppees.2016.07.002.
[23] Pina A L C B, Zandavalli R B, Oliveira R S, et al. Dew absorption by the leaf trichomes of Combretum leprosum in the Brazilian semiarid region[J]. Functional Plant Biology, 2016, 43(9): 851−861. doi: 10.1071/FP15337
[24] Eller C B, Lima A L, Oliveira R S. Foliar uptake of fog water and transport belowground alleviates drought effects in the cloud forest tree species, Drimys brasiliensis (Winteraceae)[J]. New Phytologist, 2013, 199(1): 151−162. doi: 10.1111/nph.12248.
[25] 岑宇, 刘美珍. 凝结水对干旱胁迫下羊草和冰草生理生态特征及叶片形态的影响[J]. 植物生态学报, 2017, 41(11):1199−1207. doi: 10.17521/cjpe.2017.0114. Cen Y, Liu M Z. Effects of dew on eco-physiological traits and leaf structures of Leymus chinensis and Agropyron cristatum grown under drought stress[J]. Chinese Journal of Plant Ecology, 2017, 41(11): 1199−1207. doi: 10.17521/cjpe.2017.0114.
[26] Zhuang Y L, Ratcliffe S. Relationship between dew presence and Bassia dasyphylla plant growth[J]. Journal of Arid Land, 2012, 4(1): 11−18. doi: 10.3724/SP.J.1227.2012.00011.
[27] Baguskas S A, Clemesha R E S, Loik M E. Coastal low cloudiness and fog enhance crop water use efficiency in a California agricultural system[J]. Agricultural and Forest Meteorology, 2018, 252: 109−120. doi: 10.1016/j.agrformet.2018.01.015.
[28] 林光辉. 稳定同位素生态学[M]. 北京: 高等教育出版社, 2013, 3−29. Lin G H. Stable Isotope Ecology [M]. Beijing: Higher Education Press, 2013, 3−29.
[29] West A G, Patrickson S J, Ehleringer J R. Water extraction times for plant and soil materials used in stable isotope analysis[J]. Rapid Communications in Mass Spectrometry, 2006, 20(8): 1317−1321. doi: 10.1002/rcm.2456.
[30] 刘文茹, 彭新华, 沈业杰, 等. 激光同位素分析仪测定液态水的氢氧同位素及其光谱污染修正[J]. 生态学杂志, 2013, 32(5):1181−1186. Liu W R, Peng X H, Shen Y J, et al. Measurements of hydrogen and oxygen isotopes in liquid water by isotope ratio infrared spectroscopy (IRIS) and their spectral contamination corrections[J]. Chinese Journal of Ecology, 2013, 32(5): 1181−1186.
[31] Schultz N M, Griffis T J, Lee X, et al. Identification and correction of spectral contamination in 2H/1H and 18O/16O measured in leaf, stem, and soil water[J]. Rapid Commun Mass Spectrom, 2011, 25(21): 3360−3368. doi: 10.1002/rcm.5236.
[32] Kim K, Lee X. Transition of stable isotope ratios of leaf water under simulated dew formation[J]. Plant, Cell & Environment, 2011, 34(10): 1790−1801.
[33] Guzmán-Delgado P, Earles J M, Zwieniecki M A. Insight into the physiological role of water absorption via the leaf surface from a rehydration kinetics perspective[J]. Plant, Cell & Environment, 2018, 41(8): 1886−1894.
[34] Emery N C. Foliar uptake of fog in coastal California shrub species[J]. Oecologia, 2016, 182(3): 731−742. doi: 10.1007/s00442-016-3712-4.
[35] Chamel A, Pineri M, Escoubes M. Quantitative determination of water sorption by plant cuticles[J]. Plant Cell and Environment, 1991, 14(1): 87−95. doi: 10.1111/j.1365-3040.1991.tb01374.x.
[36] Burgess S S O, Dawson T E. The contribution of fog to the water relations of Sequoia sempervirens (D. Don): foliar uptake and prevention of dehydration[J]. Plant, Cell and Environment, 2004, 27(8): 1023−1034. doi: 10.1111/j.1365-3040.2004.01207.x.
[37] 屠骊珠. 内蒙古西部地区九种旱生植物叶的解剖观察[J]. 内蒙古大学学报(自然科学版), 1982, 13(4):485−504. Tu L Z. Anatomical observations on nine xerophytes leaves in the west part of the Inner Mongolia[J]. Journal of Inner Mongolia University( Natural Science Edition), 1982, 13(4): 485−504.
[38] Yates D J, Hutley L B. Foliar uptake of water by wet leaves of Sloanea woollsii, an Australian subtropical rainforest tree[J]. Australian Journal of Botany, 1995, 43(2): 157−167. doi: 10.1071/BT9950157.
[39] Rundel P W. Water uptake by organs other than roots[J]. Physiological Plant Ecology II, 1982, 12: 111−134.
[40] Grammatikopoulus G, Manetas Y. Direct absorption of water by hairy leaves of Phlomis fruticosa and its contribution to drought avoidance[J]. Canadian Journal of Botany, 1994, 72(12): 1805−1811. doi: 10.1139/b94-222.
[41] Alvarado-Barrientos M S, Holwerda F, Asbjornsen H, et al. Suppression of transpiration due to cloud immersion in a seasonally dry Mexican weeping pine plantation[J]. Agricultural and Forest Meteorology, 2014, 186: 12−25. doi: 10.1016/j.agrformet.2013.11.002.
[42] Berry Z C, Smith W K. Cloud pattern and water relations in Picea rubens and Abies fraseri, southern Appalachian Mountains, USA[J]. Agricultural and Forest Meteorology, 2012, 162−163: 27−34. doi: 10.1016/j.agrformet.2012.04.005.
[43] 付晓玥. 阿拉善荒漠植物叶片性状研究[D]. 呼和浩特: 内蒙古大学, 2012. Fu X Y. Studies on leaf traits of Alashan desert plants[D]. Huhhot: Inner Mongolia University, 2012.
[44] 王春海. 中国藜属及近缘属植物的系统学研究[D]. 曲阜: 曲阜师范大学, 2015. Wang C H. Systematic study on Chenopodium and the related genera in China[D]. Qufu: Qufu Normal University, 2015.
[45] Goldsmith G R, Lehmann M M, Cernusak L A, et al. Inferring foliar water uptake using stable isotopes of water[J]. Oecologia, 2017, 184(4): 763−766. doi: 10.1007/s00442-017-3917-1.
[46] 范志超. 不同生境苦豆子种群生产性能与种子休眠特性研究[D]. 兰州: 兰州大学, 2016. Fan Z C. Study on productivity and seed dormancy characteristics of Sophora alopecuroides L. populations in different habitats[D]. Lanzhou: Lanzhou University, 2016.
-
期刊类型引用(5)
1. 徐金生,姜礅,严善春. Cd胁迫对银中杨幼苗金属元素代谢及其氧化应激的影响. 安徽农业大学学报. 2023(06): 1000-1005 .  百度学术
百度学术
2. 单羽,任晓宁,李雪梅. 非生物胁迫对植物碳水化合物及其代谢相关酶影响的研究进展. 安徽农业科学. 2021(20): 6-9 .  百度学术
百度学术
3. 熊露露,卢囿佐,刘月炎,彭海兰,黄兴敏,王健健. 外源锌对氮胁迫下薏苡幼苗生长及抗氧化系统的影响. 中国农学通报. 2021(36): 24-29 .  百度学术
百度学术
4. 刘景华. 不同锌胁迫浓度下杨树生长以及生物量参数的变化. 林业科技通讯. 2020(07): 73-74 .  百度学术
百度学术
5. 王月月,董效文,姜礅,狄贵秋,王沫,刘晓霞,孟昭军,严善春. 镉、锌复合胁迫对银中杨化学防御的影响. 北京林业大学学报. 2019(06): 96-101 .  本站查看
本站查看
其他类型引用(5)




 下载:
下载: